��Ŀ����
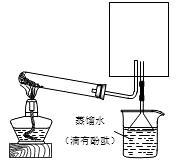
����10�֣���֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����Ӧ����ʽΪ2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺

(1��A�з�����Ӧ�Ļ�ѧ����ʽ�� �����鰱��ͨ�����õķ�����������_________ ______�� ��
(2��B���������� ���������� ��
(3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______����
(4����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D���ձ����ָij����������ĸ���ܣ���һ�������X��װ��_____________��������___________________���ڶ��������Y��װ�м�ʯ�ң�������____________________________��
 N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ���ش��������⣺
(1��A�з�����Ӧ�Ļ�ѧ����ʽ�� �����鰱��ͨ�����õķ�����������_________ ______�� ��
(2��B���������� ���������� ��
(3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______����
(4����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D���ձ����ָij����������ĸ���ܣ���һ�������X��װ��_____________��������___________________���ڶ��������Y��װ�м�ʯ�ң�������____________________________��
��1��2NH4Cl+Ca(OH)2 2NH3��+CaCl2+2H2O (1��)
2NH3��+CaCl2+2H2O (1��)
��ʪ��ĺ�ɫʯ����ֽ��1�֣�����ֽ������1�֣�
��2����ʯ�ң���1�֣���ȥ��ˮ�е�ˮ������1�֣�
��3����ɫ������ɺ�ɫ��1�֣�����ԭ��1�֣�
��4����ˮ����ͭ��1�֣���������1�֣�
��ֹE�е�ˮ������������x��Ӱ��ˮ�ļ��顣��1�֣�
 2NH3��+CaCl2+2H2O (1��)
2NH3��+CaCl2+2H2O (1��)��ʪ��ĺ�ɫʯ����ֽ��1�֣�����ֽ������1�֣�
��2����ʯ�ң���1�֣���ȥ��ˮ�е�ˮ������1�֣�
��3����ɫ������ɺ�ɫ��1�֣�����ԭ��1�֣�
��4����ˮ����ͭ��1�֣���������1�֣�
��ֹE�е�ˮ������������x��Ӱ��ˮ�ļ��顣��1�֣�
��1��A������ȡ�����ģ����Է���ʽΪ2NH4Cl+Ca(OH)2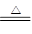 2NH3��+CaCl2+2H2O����������ˮ�Լ��ԣ����Ȼ��ⷴӦð���̣��ݴ˿��Լ��顣
2NH3��+CaCl2+2H2O����������ˮ�Լ��ԣ����Ȼ��ⷴӦð���̣��ݴ˿��Լ��顣
��2��������ͭ��Ӧ�İ���Ӧ���Ǹ���ģ�����B���ü�ʯ�ҳ�ȥ�����е�ˮ������
��3���������л�ԭ�ԣ��ڼ��ȵ������£��ܻ�ԭ����ͭ������ͭ��������ˮ�����������Ǻ�ɫ������ɺ�ɫ��
��4������ˮ����������ˮ����ͭ����ˮ�������ɫ������װ��E��ˮ�������ܽ������ܡ�Ӱ��ˮ�ļ��飬�������þ��Ƿ�ֹE�е�ˮ������������x��Ӱ��ˮ�ļ��顣
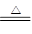 2NH3��+CaCl2+2H2O����������ˮ�Լ��ԣ����Ȼ��ⷴӦð���̣��ݴ˿��Լ��顣
2NH3��+CaCl2+2H2O����������ˮ�Լ��ԣ����Ȼ��ⷴӦð���̣��ݴ˿��Լ��顣��2��������ͭ��Ӧ�İ���Ӧ���Ǹ���ģ�����B���ü�ʯ�ҳ�ȥ�����е�ˮ������
��3���������л�ԭ�ԣ��ڼ��ȵ������£��ܻ�ԭ����ͭ������ͭ��������ˮ�����������Ǻ�ɫ������ɺ�ɫ��
��4������ˮ����������ˮ����ͭ����ˮ�������ɫ������װ��E��ˮ�������ܽ������ܡ�Ӱ��ˮ�ļ��飬�������þ��Ƿ�ֹE�е�ˮ������������x��Ӱ��ˮ�ļ��顣

��ϰ��ϵ�д�
�����Ŀ