��Ŀ����
�������ʶ�ʵ�̽����������ֻ��ʣ��ֱ�������ء��Ȼ��ء�̼����李��Ȼ�狀�����李�
(1)��һ������ȡ���ֻ��ʸ�10 g���ֱ���ϸ��
(2)�ڶ���������狀���ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ�ǣ� _______________________________
________________________________________________________________________��
(3)����������ȡ������θ���������ʢ����֧�Թ��У��������������ᣬ�������������________________________________________________________________________��
�����ݷų�����________����Ӧ�Ļ�ѧ����ʽ____________________________________
________________________________________________________________________��
(4)���IJ�����ȡ�Ȼ�狀������������ν���ʶ��д��ʶ��ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(5)���岽��ȡ���ּ��Σ��ֱ����Թ��������Һ�������뼸���Ȼ�����Һ�����ɰ�ɫ�����ļ�����________������������ļ�����________��
(2)2NH4NO3��Ca(OH)2===Ca(NO3)2��2NH3����2H2O��
(3)�Ȼ�狀�����李�̼����李�NH4HCO3��HCl===NH4Cl��CO2����H2O
(4)NH4Cl��AgNO3===AgCl����NH4NO3
(5)K2SO4��KCl
��������
�����������2������ܺ�ǿ�Ӧ�ų���������������狀���ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ��2NH4NO3��Ca(OH)2===Ca(NO3)2��2NH3����2H2O��
��3���Ȼ�狀�������������Ӧ����������������Ȼ�狀�����泥�̼������ܺ����ᷴӦ�ų�CO2���壬��Ӧ�Ļ�ѧ����ʽ��NH4HCO3��HCl===NH4Cl��CO2����H2O��
��4�����������ӿ����������ữ����������Һ����Ӧ�Ļ�ѧ����ʽ��NH4Cl��AgNO3===AgCl����NH4NO3��
��5��������ܺ��Ȼ�����Ӧ���ɰ�ɫ�������ᱵ�����Ȼ��ز��ܣ��ݴ˿��Լ���
���㣺�������ʵļ��顢����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ��ע�ؿ���ѧ���������⡢����������������������ѧ����Ҫ��ȷ�ڽ������ʵļ���ʱ��Ҫ�������ʵ��������ʺ�������Ӧ��ѡ���ʵ����Լ��ͷ�����ȷ�۲췴Ӧ�е�������������ɫ�ı仯�����������ɺ��ܽ⡢����IJ�������ζ���������ɫ�ȣ������жϡ���������֤���ɡ�

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
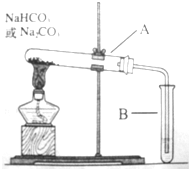 ijУ��ѧ��ȤС�飬ͨ������װ��̽��Na2CO3��NaHCO3�����ȶ��ԣ�������µ�̽��������������벢��ɸ�̽��������
ijУ��ѧ��ȤС�飬ͨ������װ��̽��Na2CO3��NaHCO3�����ȶ��ԣ�������µ�̽��������������벢��ɸ�̽��������