��Ŀ����
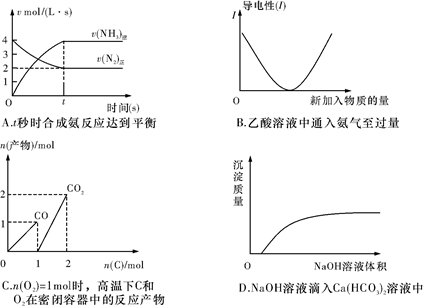
��6�֣�������ij���ʵ�ת����Ϊ���ĵ��ĸ����ʵ����ʵ���ռ��ʼʱ�����ʵ�ԭ���ʵ����İٷֱȡ���֪�ϳɰ���ӦΪN2(g) + 3H2(g)  2NH3(g).��һ���Ϊ10L���ݻ�������ܱ������з���1mol N2��3mol H2 ,��һ�������·�����Ӧ����4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol.����
2NH3(g).��һ���Ϊ10L���ݻ�������ܱ������з���1mol N2��3mol H2 ,��һ�������·�����Ӧ����4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol.����
��1��������ת����
��2����������ʾƽ����Ӧ�����Ƕ��٣�
��3��ƽ��״̬ʱ���������ʵ���Ũ�ȡ�
 2NH3(g).��һ���Ϊ10L���ݻ�������ܱ������з���1mol N2��3mol H2 ,��һ�������·�����Ӧ����4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol.����
2NH3(g).��һ���Ϊ10L���ݻ�������ܱ������з���1mol N2��3mol H2 ,��һ�������·�����Ӧ����4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol.������1��������ת����
��2����������ʾƽ����Ӧ�����Ƕ��٣�
��3��ƽ��״̬ʱ���������ʵ���Ũ�ȡ�
��1��30% ��2��0.0225 mol��(L��min)-1 ��3��0.21 mol��L-
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 5C02 (g)+H2 (g)��H>0
5C02 (g)+H2 (g)��H>0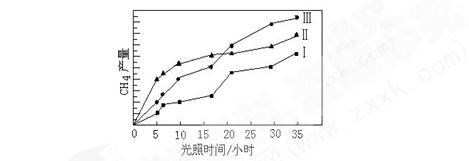
 CO(g)+3H2(g)���÷�Ӧ�ġ�H="+206" kJ?mol-1
CO(g)+3H2(g)���÷�Ӧ�ġ�H="+206" kJ?mol-1 ��������λ��Ч���֣���
��������λ��Ч���֣���