��Ŀ����
(12��)ʵ���ҿ������̿���Ҫ�ɷ�ΪMnO2���Ʊ�KMnO4���������£����̿����������KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl����ˮ�ܽ⣬��ȥ��������Һ�ữ��K2MnO4ת��ΪMnO2 ��KMnO4����ȥMnO2������Ũ����Һ���ᾧ�õ�����ɫ����״KMnO4����ش�
��1�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ��
������������������������������������������������������������������������
��2��K2MnO4�Ʊ�KMnO4�����ӷ���ʽ������������������������������������
��3������2.5g���̿�MnO280������������ʵ�飬����KMnO4�����۲�����
��4��KMnO4�����ȵľ������ữ��Na2C2O4��Ӧ����Mn2+��CO2���÷�Ӧ�Ļ�ѧ����ʽ������������������������������������������������������������������������
��5�������Ƶõ�KMnO4��Ʒ0.165g��ǡ����0.335g��Na2C2O4��Ӧ��ȫ�������KMnO4�Ĵ��ȡ�
(12��)(1��3MnO2+6KOH+KClO3 3K2MnO4+KCl+3H2O(2��)
3K2MnO4+KCl+3H2O(2��)
��2��3Mn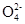 +4H+ = MnO2��+2Mn
+4H+ = MnO2��+2Mn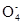 +2H2O(2��)
+2H2O(2��)
��3��������������ѧ����ʽ��֪����Ӧ����������ļ�����ϵΪ��(3��)
 MnO2
K2MnO4
MnO2
K2MnO4
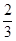 KMnO4
KMnO4
87
158��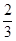
2.5��80% x
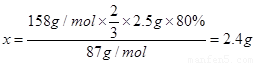
��KMnO4�����۲�����2.4g
��4��2KMnO4+5Na2C2O4+8H2SO4 K2SO4+2MnSO4+5Na2SO4+10CO2��+8H2O(2��)
K2SO4+2MnSO4+5Na2SO4+10CO2��+8H2O(2��)
��5�����KMnO4�Ĵ���Ϊy�����ݻ�ѧ����ʽ��֪(3��)
 KMnO4������Na2C2O4
KMnO4������Na2C2O4
 ��158 134
��158 134
0.165 �� y 0.335
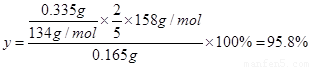
����������
