��Ŀ����
��1��(2��)�����й�ʵ��������жϲ���ȷ���� ________������ţ���ѡ�۷֣���
A������һ�����ʵ���Ũ����Һ������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫС
B������Ũ����մ��Ƥ���ϣ�����������������Һ��ϴ
C��Һ���ж����ӷ�,��ʢ����ĥ�ڵ�ϸ��ƿ��,��ˮ�Ᵽ��
D��100 mL����ƿ����������95 mL 0.1 mol/L NaCl��Һ
E������ƽ���������и���һ�Ű�ֽ���ɽ�NaOH������ڰ�ֽ�ϳ���
(2) (2��)˫��ˮ��H2O2���Ǽ����ĵ���ʣ� H2O2��Һ�������ԡ�����H2O2���ɶ�Ԫ���ᣬ��д������ˮ�еĵ��뷽��ʽ ,
(3) (4��)��������������Ӧ����1molˮ����������241.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ ����1gˮ����ת����Һ̬ˮ����2.444 kJ����H2��ȼ���Ȧ�H= kJ��mol-1
��8�֣�ABE ��2�֣� H2O2H++HO2- HO2-
H++O22-��2�֣�
2H2(g)+O2(g)=2H2O(g) ��H=-483.6 kJ��mol-1 ��2�֣� -285.8����2�֣�
����:

��6�֣�����⣺
��1��(2��)����ʵ���������ȷ���� ������ĸ���ţ���
| A���ڴ������ڵ������£�������ˮ������Ӧ��������ɫ����ˮ�ص�Һ���屽�� |
| B����ͭ˿�������״���ھƾ����ϼ��ȱ�ں���������ʢ����ˮ�Ҵ����Թ��У�����Ҵ�����Ϊ��ȩ��ʵ�顣 |
| C�����к�������ˮ�ɼ�����ʯ���������Ƶ���ˮ�Ҵ��� |
| D���г�һ���Ա�������϶������¿�ʱ����������������ɡ����ε��ᴿ��ʵ�� |
F����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����á���Һ��ֲ�μ���������Һ��
G��ʵ��������ʯ��ʱ�¶ȼ�ˮ�������ʯ���в����¶ȣ��ռ�60�桫150����ֵõ����͡�
��2����2�֣�
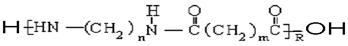 �ĵ���Ϊ___________
�ĵ���Ϊ___________ άͨ����һ����ʴ�����͡��߳����ͺ����ܶ��ر�õķ������Ľṹ��ʽΪ��
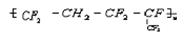 �ϳ����ĵ���Ϊ________________________
�ϳ����ĵ���Ϊ________________________ 

 2SO3(g) ��H=��190 kJ��mo1��1
2SO3(g) ��H=��190 kJ��mo1��1