��Ŀ����
�ɱ��������Ģ١���������Ϣ�������ѧ֪ʶ���ش��������⣮��
 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ���� ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺

��1��д�������ϩ�����ֽ�ĸ��ֲ���Ľṹ��ʽ �����ǵ����ʵ���֮��Ϊ ��
��2��a molij��CnH2n-2���÷�������-C��C-����
 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ��� ��b mol����a��b�Ĵ�����ϵ�ǣ� �� ��
��b mol����a��b�Ĵ�����ϵ�ǣ� �� ����3��д���ɻ�������
 �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ��
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ����4��ij��A�Ļ�ѧʽΪC10H16��A�������ֽ�ɵõ������ʵ��������ֲ����ṹ��ʽ�ֱ�ΪHCHO��
 ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B ��
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B ����5�����㾫���з��뵽һ�ֻ�����[A]��C10H16����1mol[A]�ڴ�����ʱ������2mol������1mol[A]������������Ӧ�ټ�п��ˮ��ɵõ�1mol��ͪ[��CH3��2C=O]��1mol3��6-���ʻ���ȩ
 �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ ��
��6��һ�����Ļ�ѧʽ��ΪC4H8�IJ��������Ļ�����壬�������ֽ������8.7gͪ��0.45mol��ȩ�����м�ȩ��0.21mol������ͨ������ش��������⣺
��a��������������Щ���ʣ�д���ṹ��ʽ ��
��b����������к��ļ�������д�ṹ��ʽ���������ʵ���֮��Ϊ���٣� �� ��
���𰸡���������1���ɷ�Ӧ��Ϣ�ڿ�֪�������ϩ�����ֽ�����HCHO��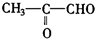 �����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1��
�����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1��
��2����CnH2n-2���÷�������-C��C-����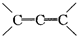 �ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ���
�ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ���
��3�� ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ������������
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ������������
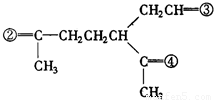
��4��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��A�������ӳ�����B��B�Ļ�ѧʽΪC10H20�������ں�����Ԫ̼������A�к���1����Ԫ����2��C=C˫���������Ƚ�������IJ��︴ԭ��ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�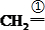 ��
�� �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽��
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽��
��5��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��1mol[A]�ڴ�����ʱ������2mol�����������к���2��C=C˫������A�ķ����л�����1��������Ԫ������Ԫ�����ȶ�����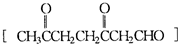 ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����
ȥ������ϵ����������µ�˫��λ�ñ�ţ�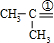 ��
��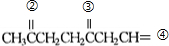 ���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д��
���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д��
��6��һ�����Ļ�ѧʽ��ΪC4H8�IJ��������Ļ�����壬��������ϩ���������ܵĽṹ��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�������������ֱ�����ӦCH3CH2CH=CH2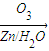 CH3CH2CHO+HCHO��CH3CH=CHCH3
CH3CH2CHO+HCHO��CH3CH=CHCH3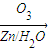 CH3CHO����CH3��2C=CH2
CH3CHO����CH3��2C=CH2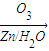 ��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
����n= �����ͪ�����ʵ�������Ϸ���ʽ��CH3��2C=CH2
�����ͪ�����ʵ�������Ϸ���ʽ��CH3��2C=CH2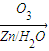 ��CH3��2CO+HCHO���㣨CH3��2C=CH2�����������ɵ�HCHO�����ʵ�������������CH3CH2CH=CH2���ɵ�CH3CH2CHO��HCHO�����ʵ���������ȩ���ܵ����ʵ���������ȩ�����ʵ�������Ϸ���ʽ������������ʵ������ݴ˽��
��CH3��2CO+HCHO���㣨CH3��2C=CH2�����������ɵ�HCHO�����ʵ�������������CH3CH2CH=CH2���ɵ�CH3CH2CHO��HCHO�����ʵ���������ȩ���ܵ����ʵ���������ȩ�����ʵ�������Ϸ���ʽ������������ʵ������ݴ˽��
����⣺��1���ɷ�Ӧ��Ϣ�ڿ�֪�������ϩ�����ֽⷽ��ʽΪCH2=C��CH3��CH=CH2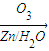 2HCHO+
2HCHO+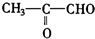 ���ɷ���ʽ��֪HCHO��
���ɷ���ʽ��֪HCHO��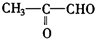 �������ʵ���֮��Ϊ2��1��
�������ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��HCHO��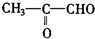 ��2��1��
��2��1��
��2����CnH2n-2���÷�������-C��C-����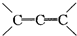 �ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol
�ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol ����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol
����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol ����b=2a���ʴ�Ϊ��b=4a��b=2a��
����b=2a���ʴ�Ϊ��b=4a��b=2a��
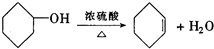
��3��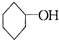 ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ��
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ��
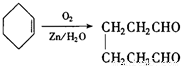
�� ��
��
�� ��
��
��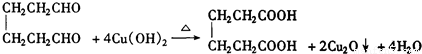 ��
��
�� ��
��
�ʴ�Ϊ����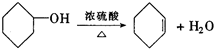 ��
��
��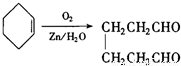 ��
��
��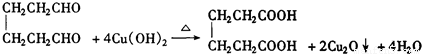 ��
��
�� ��
��
��4��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��A�������ӳ�����B��B�Ļ�ѧʽΪC10H20�������ں�����Ԫ̼������A�к���1����Ԫ����2��C=C˫���������Ƚ�������IJ��︴ԭ��ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�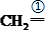 ��
��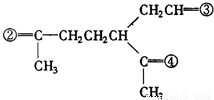 �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ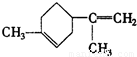 ��BΪ
��BΪ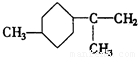 ��
��
�ʴ�Ϊ��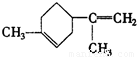 ��
�� ��
��
��5��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��1mol[A]�ڴ�����ʱ������2mol�����������к���2��C=C˫������A�ķ����л�����1��������Ԫ������Ԫ�����ȶ�����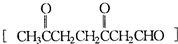 ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����
ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�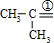 ��
��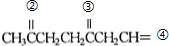 ���ڢ������γ���Ԫ�����٢������γɲ�������AΪ
���ڢ������γ���Ԫ�����٢������γɲ�������AΪ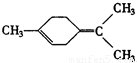 ��
��
�ʴ�Ϊ��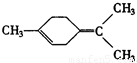 ��
��
��6����a��һ�����Ļ�ѧʽ��ΪC4H8�IJ��������Ļ�����壬��������ϩ���������ܵĽṹ��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�������������ֱ�����ӦCH3CH2CH=CH2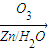 CH3CH2CHO+HCHO��CH3CH=CHCH3
CH3CH2CHO+HCHO��CH3CH=CHCH3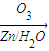 CH3CHO����CH3��2C=CH2
CH3CHO����CH3��2C=CH2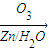 ��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO���ʴ�Ϊ��CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO���ʴ�Ϊ��CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
��b���ɣ�a���ķ�����֪����������к���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3��
��ͪ�����ʵ���Ϊ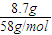 =0.15mol����Ϸ���ʽ��CH3��2C=CH2
=0.15mol����Ϸ���ʽ��CH3��2C=CH2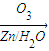 ��CH3��2CO+HCHO��֪��CH3��2C=CH2�����ʵ���Ϊ0.15mol���������ɵ�HCHO�����ʵ���Ϊ0.15mol���ɷ���ʽ��֪��CH3CH2CH=CH2���ɵ�HCHO�����ʵ���Ϊ0.21mol-0.15mol=0.06mol����CH3CH2CH=CH2�����ʵ���Ϊ0.06mol��CH3CH2CHO�����ʵ���Ϊ0.06mol������ȩ�����ʵ���Ϊ0.45mol-0.21mol-0.06mol=0.18mol���ɷ���ʽ��֪CH3CH=CHCH3�����ʵ���Ϊ0.09mol���ʡ���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�����ʵ���֮��Ϊ0.15mol��0.06mol��0.09mol=5��2��3��
��CH3��2CO+HCHO��֪��CH3��2C=CH2�����ʵ���Ϊ0.15mol���������ɵ�HCHO�����ʵ���Ϊ0.15mol���ɷ���ʽ��֪��CH3CH2CH=CH2���ɵ�HCHO�����ʵ���Ϊ0.21mol-0.15mol=0.06mol����CH3CH2CH=CH2�����ʵ���Ϊ0.06mol��CH3CH2CHO�����ʵ���Ϊ0.06mol������ȩ�����ʵ���Ϊ0.45mol-0.21mol-0.06mol=0.18mol���ɷ���ʽ��֪CH3CH=CHCH3�����ʵ���Ϊ0.09mol���ʡ���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�����ʵ���֮��Ϊ0.15mol��0.06mol��0.09mol=5��2��3��
�ʴ�Ϊ����CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3��5��2��3��
��������Ŀ�ۺ��Խϴ������ϴ��漰�л�����ƶ���ϳɡ������ŵ����ʡ�ͬ���칹����д���л�������Լ��Է�Ӧ��Ϣ�����������ȣ��Ѷ��еȣ��������ķ�Ӧ��Ϣ�ǽ���Ĺؼ���
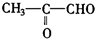 �����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1��
�����ݷ���ʽ��֪�������ʵ���֮��Ϊ2��1����2����CnH2n-2���÷�������-C��C-����
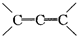 �ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ���
�ṹ�������л���Ϊ��״��ϩ����ϩ�����ݴ˽����Ϣ�����3��
 ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ������������
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ��������������4��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��A�������ӳ�����B��B�Ļ�ѧʽΪC10H20�������ں�����Ԫ̼������A�к���1����Ԫ����2��C=C˫���������Ƚ�������IJ��︴ԭ��ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�
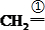 ��
�� �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽��
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ݴ˽����5��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��1mol[A]�ڴ�����ʱ������2mol�����������к���2��C=C˫������A�ķ����л�����1��������Ԫ������Ԫ�����ȶ�����
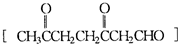 ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����ȥ������ϵ����������µ�˫��λ�ñ�ţ�
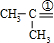 ��
��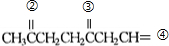 ���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д��
���ڢ������γ���Ԫ�����٢������γɲ������ݴ���д����6��һ�����Ļ�ѧʽ��ΪC4H8�IJ��������Ļ�����壬��������ϩ���������ܵĽṹ��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�������������ֱ�����ӦCH3CH2CH=CH2
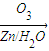 CH3CH2CHO+HCHO��CH3CH=CHCH3
CH3CH2CHO+HCHO��CH3CH=CHCH3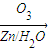 CH3CHO����CH3��2C=CH2
CH3CHO����CH3��2C=CH2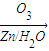 ��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO������n=
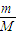 �����ͪ�����ʵ�������Ϸ���ʽ��CH3��2C=CH2
�����ͪ�����ʵ�������Ϸ���ʽ��CH3��2C=CH2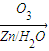 ��CH3��2CO+HCHO���㣨CH3��2C=CH2�����������ɵ�HCHO�����ʵ�������������CH3CH2CH=CH2���ɵ�CH3CH2CHO��HCHO�����ʵ���������ȩ���ܵ����ʵ���������ȩ�����ʵ�������Ϸ���ʽ������������ʵ������ݴ˽��
��CH3��2CO+HCHO���㣨CH3��2C=CH2�����������ɵ�HCHO�����ʵ�������������CH3CH2CH=CH2���ɵ�CH3CH2CHO��HCHO�����ʵ���������ȩ���ܵ����ʵ���������ȩ�����ʵ�������Ϸ���ʽ������������ʵ������ݴ˽������⣺��1���ɷ�Ӧ��Ϣ�ڿ�֪�������ϩ�����ֽⷽ��ʽΪCH2=C��CH3��CH=CH2
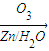 2HCHO+
2HCHO+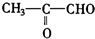 ���ɷ���ʽ��֪HCHO��
���ɷ���ʽ��֪HCHO��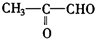 �������ʵ���֮��Ϊ2��1��
�������ʵ���֮��Ϊ2��1���ʴ�Ϊ��HCHO��
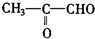 ��2��1��
��2��1����2����CnH2n-2���÷�������-C��C-����
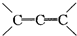 �ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol
�ṹ������Ϊ��״��ϩ�������Ӻ���2��C=C˫�����ɷ�Ӧ��Ϣ��֪��1mol������������4mol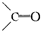 ����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol
����b=4a����Ϊ��ϩ���������к���1��C=C˫������1mol������������2mol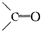 ����b=2a���ʴ�Ϊ��b=4a��b=2a��
����b=2a���ʴ�Ϊ��b=4a��b=2a����3��
 ������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ��
������ȥ��Ӧ�������Ļ���ϩ������ϩ�������O3����Ӧ������п�۴�����ˮ������OHCCH2CH2CH2CH2CHO��������������ͭ��Ӧ����HOOCCH2CH2CH2CH2COOH��HOOCCH2CH2CH2CH2COOH���Ҵ���1��2��Ӧ���ɼ�������������ϳɼ�����������ĸ�����Ӧ����ʽΪ����
 ��
����
 ��
����
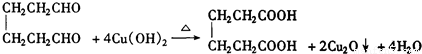 ��
����
 ��
���ʴ�Ϊ����
 ��
����
 ��
����
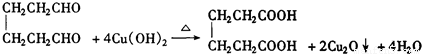 ��
����
 ��
����4��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��A�������ӳ�����B��B�Ļ�ѧʽΪC10H20�������ں�����Ԫ̼������A�к���1����Ԫ����2��C=C˫���������Ƚ�������IJ��︴ԭ��ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�
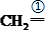 ��
�� �����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ
�����������ں͢��Ƚӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬�ۢܽӳɻ������ں͢۽ӳɻ����ٺ͢��ٽ�Ϊ�����������γ���Բ�����������⣬��AΪ ��BΪ
��BΪ ��
���ʴ�Ϊ��
 ��
�� ��
����5��A�ķ���ʽΪC10H16�������Ͷ�Ϊ3��1mol[A]�ڴ�����ʱ������2mol�����������к���2��C=C˫������A�ķ����л�����1��������Ԫ������Ԫ�����ȶ�����
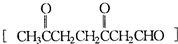 ���֪�������д�����Ԫ����
���֪�������д�����Ԫ����ȥ������ϵ������ҽ����µ�˫��λ�ñ�ţ�
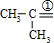 ��
��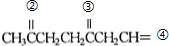 ���ڢ������γ���Ԫ�����٢������γɲ�������AΪ
���ڢ������γ���Ԫ�����٢������γɲ�������AΪ ��
���ʴ�Ϊ��
 ��
����6����a��һ�����Ļ�ѧʽ��ΪC4H8�IJ��������Ļ�����壬��������ϩ���������ܵĽṹ��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�������������ֱ�����ӦCH3CH2CH=CH2
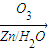 CH3CH2CHO+HCHO��CH3CH=CHCH3
CH3CH2CHO+HCHO��CH3CH=CHCH3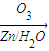 CH3CHO����CH3��2C=CH2
CH3CHO����CH3��2C=CH2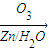 ��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO���ʴ�Ϊ��CH3CH2CHO��CH3CHO��HCHO����CH3��2CO��
��CH3��2CO+HCHO���ʾ����������ֽ�����ò���CH3CH2CHO��CH3CHO��HCHO����CH3��2CO���ʴ�Ϊ��CH3CH2CHO��CH3CHO��HCHO����CH3��2CO����b���ɣ�a���ķ�����֪����������к���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3��
��ͪ�����ʵ���Ϊ
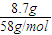 =0.15mol����Ϸ���ʽ��CH3��2C=CH2
=0.15mol����Ϸ���ʽ��CH3��2C=CH2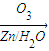 ��CH3��2CO+HCHO��֪��CH3��2C=CH2�����ʵ���Ϊ0.15mol���������ɵ�HCHO�����ʵ���Ϊ0.15mol���ɷ���ʽ��֪��CH3CH2CH=CH2���ɵ�HCHO�����ʵ���Ϊ0.21mol-0.15mol=0.06mol����CH3CH2CH=CH2�����ʵ���Ϊ0.06mol��CH3CH2CHO�����ʵ���Ϊ0.06mol������ȩ�����ʵ���Ϊ0.45mol-0.21mol-0.06mol=0.18mol���ɷ���ʽ��֪CH3CH=CHCH3�����ʵ���Ϊ0.09mol���ʡ���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�����ʵ���֮��Ϊ0.15mol��0.06mol��0.09mol=5��2��3��
��CH3��2CO+HCHO��֪��CH3��2C=CH2�����ʵ���Ϊ0.15mol���������ɵ�HCHO�����ʵ���Ϊ0.15mol���ɷ���ʽ��֪��CH3CH2CH=CH2���ɵ�HCHO�����ʵ���Ϊ0.21mol-0.15mol=0.06mol����CH3CH2CH=CH2�����ʵ���Ϊ0.06mol��CH3CH2CHO�����ʵ���Ϊ0.06mol������ȩ�����ʵ���Ϊ0.45mol-0.21mol-0.06mol=0.18mol���ɷ���ʽ��֪CH3CH=CHCH3�����ʵ���Ϊ0.09mol���ʡ���CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3����CH3��2C=CH2�����ʵ���֮��Ϊ0.15mol��0.06mol��0.09mol=5��2��3���ʴ�Ϊ����CH3��2C=CH2��CH3CH2CH=CH2��CH3CH=CHCH3��5��2��3��
��������Ŀ�ۺ��Խϴ������ϴ��漰�л�����ƶ���ϳɡ������ŵ����ʡ�ͬ���칹����д���л�������Լ��Է�Ӧ��Ϣ�����������ȣ��Ѷ��еȣ��������ķ�Ӧ��Ϣ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
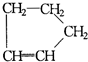 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ���� ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺


 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ��� ��b mol����a��b�Ĵ�����ϵ�ǣ�
��b mol����a��b�Ĵ�����ϵ�ǣ� �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ��
�� ��
�� ��
��
 ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA

 �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ 
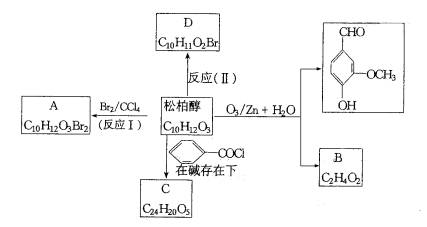

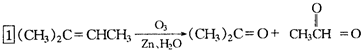
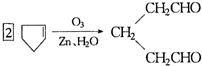
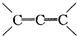

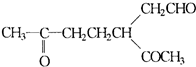
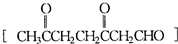
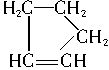 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ�
������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ�
 �������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ�
�������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ� ���ϳɼ���ȩ��
���ϳɼ���ȩ�� ���ĸ���ת����ϵ��
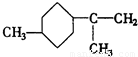
���ĸ���ת����ϵ�� ��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______��
��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______��