��Ŀ����
�ɱ���������������Ϣ�������ѧ֪ʶ���ش���������С�⣺��.
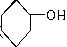
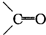
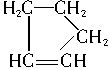 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ�
������ϩ�Ļ�ѧ���ʸ�ϩ�������ơ���.�л�����ϩ���ɷ������·�Ӧ��
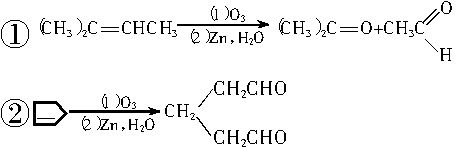
��1��д�������ϩ
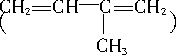 �������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ�
�������ֽ����ø��ֲ���Ľṹ��ʽ�������ʵ���֮�ȡ���2��д���ɻ�������
 ���ϳɼ���ȩ��
���ϳɼ���ȩ��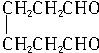 ���ĸ���ת����ϵ��
���ĸ���ת����ϵ����3��ij���ķ���ʽΪC10H16���������ֽ�ɵõ������ʵ��������ֲ����ṹ��ʽ�ֱ�ΪHCHO��
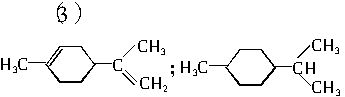
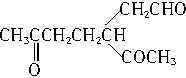 ��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______��
��A��������ò���B��B�ķ���ʽΪC10H20���������ݱ���������B�ں���һ����Ԫ̼������д��A��B�Ľṹ��ʽ������ע�����ƣ���AΪ____��BΪ_______��
��1��HCHO��CH3 COCHO��2��1
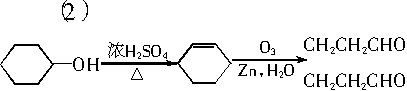
�����������

��ϰ��ϵ�д�
�����Ŀ
 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����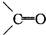 ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺


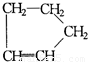
 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ���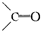 ��b mol����a��b�Ĵ�����ϵ�ǣ�
��b mol����a��b�Ĵ�����ϵ�ǣ� �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ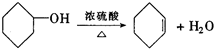 ��
��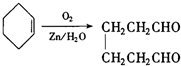 ��
�� ��
��
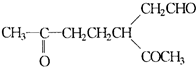 ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA

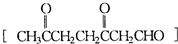 �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ 
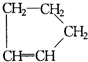

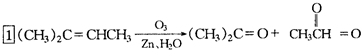
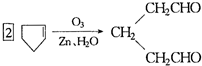
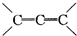

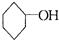

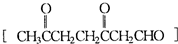
 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ ����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ����
����ϩ���Ļ�ѧ���ʸ�ϩ�����ƣ� ���л��������е�ϩ�����Ը�������O3����Ӧ������п�۴�����ˮ�⼴��ԭ�е�ϩ�����ѣ����Ѵ����˵�̼ԭ�Ӹ����1����ԭ�Ӷ�����ȩ����-CHO����ͪ���� ������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺
������������Ӧ����һ�𣬳�Ϊ��ϩ���ij����ֽ⡱�����磺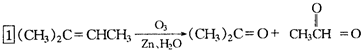
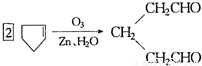
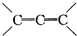 �ṹ�������������ֽ����л������к����ʻ���
�ṹ�������������ֽ����л������к����ʻ���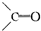 ��b mol����a��b�Ĵ�����ϵ�ǣ� �� ��
��b mol����a��b�Ĵ�����ϵ�ǣ� �� ��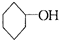 �����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ ��
�����Ҵ�Ϊ�л�ԭ�ϣ��ϳɼ�����������ĸ�����Ӧ����ʽ �� ��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B ��
��A���������ò���B��B�Ļ�ѧʽΪC10H20���������ݱ�����B�����ں�����Ԫ̼������д��A��B�Ľṹ��ʽA ��B ��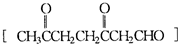 �����Ʋ��[A]�ĽṹʽΪ
�����Ʋ��[A]�ĽṹʽΪ 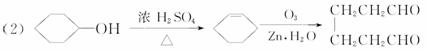 �ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ
�ǻ���ϩ�Ľṹ��ʽ���ɽ�һ����дΪ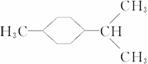

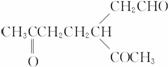 ��A��������ò���B��B�ķ���ʽ��C10H20���������ݱ���������B�ں�����Ԫ̼������д��A��B�Ľṹ��ʽ(����ע������)��A��_____________��B��_____________��
��A��������ò���B��B�ķ���ʽ��C10H20���������ݱ���������B�ں�����Ԫ̼������д��A��B�Ľṹ��ʽ(����ע������)��A��_____________��B��_____________��