��Ŀ����
ijͬѧ�����й��������ʵ����֪����Щ��ʵ�ֱ�������������Щ���ʣ�
����ո�
��1�����õ������Ի�ɫ�����ʣ�______��Ӧ����ʽΪ______
��2�����ơ��������������¿�ʢŨ������ʣ�______
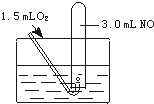
��3��ͭƬ�����������У���Һ�������Թܿ��к���ɫ������������ʣ�______��______���йط�Ӧ�ķ���ʽΪ______
��4��̼���ʷ����ȵ�Ũ�����в��������ĺ���ɫ���壺���ʣ�______���йط�Ӧ����ʽΪ______
��5��Ũ���᳨�ڷ����ڿ����У���������٣�����______��
����ո�
��1�����õ������Ի�ɫ�����ʣ�______��Ӧ����ʽΪ______
��2�����ơ��������������¿�ʢŨ������ʣ�______
��3��ͭƬ�����������У���Һ�������Թܿ��к���ɫ������������ʣ�______��______���йط�Ӧ�ķ���ʽΪ______
��4��̼���ʷ����ȵ�Ũ�����в��������ĺ���ɫ���壺���ʣ�______���йط�Ӧ����ʽΪ______
��5��Ũ���᳨�ڷ����ڿ����У���������٣�����______��
��1������ȶ��ֽ����ɶ���������������������������ʻ�ɫ����Ӧ����ʽΪ4HNO3�T4NO2��+O2��+2H2O��
�ʴ�Ϊ�����ȶ��ԣ�4HNO3�T4NO2��+O2��+2H2O��
��2��Ũ�������ǿ�����ԣ�����ʹAl��Fe�����ۻ�����ֹ�ڲ�����������Ӧ�������ơ��������������¿�ʢŨ���ᣬ
�ʴ�Ϊ��ǿ�����ԣ�
��3��ͭƬ�����������У���Һ�������Թܿ��к���ɫ���������˵����������ͭ��NO��ͬʱ����ˮ���������ǿ�����������ԣ���Ӧ����ʽΪ��3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��ǿ�����ԡ����ԣ�3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O��
��4��̼���ʷ����ȵ�Ũ�����в��������ĺ���ɫ���壬������Ϊ�������������ݵ���ת���غ��֪�������ɶ�����̼��ͬʱ����ˮ������ֻ����ǿ�����ԣ���Ӧ����ʽΪ��C+4HNO3
CO2��+4NO2��+2H2O��
�ʴ�Ϊ��ǿ�����ԣ�C+4HNO3
CO2��+4NO2��+2H2O��
��5������Ũ�����ӷ���Ũ���᳨�ڷ����ڿ����У���������٣�
�ʴ�Ϊ���ӷ��ԣ�
�ʴ�Ϊ�����ȶ��ԣ�4HNO3�T4NO2��+O2��+2H2O��
��2��Ũ�������ǿ�����ԣ�����ʹAl��Fe�����ۻ�����ֹ�ڲ�����������Ӧ�������ơ��������������¿�ʢŨ���ᣬ
�ʴ�Ϊ��ǿ�����ԣ�
��3��ͭƬ�����������У���Һ�������Թܿ��к���ɫ���������˵����������ͭ��NO��ͬʱ����ˮ���������ǿ�����������ԣ���Ӧ����ʽΪ��3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��ǿ�����ԡ����ԣ�3Cu+8HNO3�T3Cu��NO3��2+2NO��+4H2O��
��4��̼���ʷ����ȵ�Ũ�����в��������ĺ���ɫ���壬������Ϊ�������������ݵ���ת���غ��֪�������ɶ�����̼��ͬʱ����ˮ������ֻ����ǿ�����ԣ���Ӧ����ʽΪ��C+4HNO3
| ||
�ʴ�Ϊ��ǿ�����ԣ�C+4HNO3
| ||
��5������Ũ�����ӷ���Ũ���᳨�ڷ����ڿ����У���������٣�
�ʴ�Ϊ���ӷ��ԣ�

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ