��Ŀ����
ij����R�������ķ����ԣ���ԭ�Ӻ�������������������43��������ɵĹ��嵥��A����һ�������£��ܶ�Ϊ6.88g��cm-3����X�����о������������ڱ߳�Ϊ1.0��10-7cm���������к���20��ԭ�ӡ�R�ڻ�ѧ��Ӧ�г�����Ϊ+2��+4���ּ�̬������Һ�У�R2+�ȶ���R4+ȴ��ǿ�����ԣ��ɽ�NO����ΪHNO3��������R3O4��������Fe3O4��Щ���ơ�R��һ����������RС2��ͬλ�ؽ�Ϊ�ȶ����������γɺϽ��ڳ�ʪ�Ļ��������γ����⡣R�ĵ��ʼ��仯�����ڹ�ũҵ������ҽ����������ѧ�����ȸ��������кܶ���Ҫ��;����ش���1��Rԭ�ӵ�Ħ������Ϊ________��
��2��RԪ�ش���Ԫ�����ڱ��е�________����________�塣
��3������������RO2�ӵ�FeCl2��Һ�У��Ƿ�ᷢ����Ӧ________�����ܣ���Ӧ�����ӷ���ʽΪ________��
��4����R3O4����ǿ�����Զ���������������Ϳ�ϣ�Ϳ�ڸ������棬�������γ���ʴ�Ķۻ��㡣R3O4����������ϡ���ᷴӦ������R�������R
�𰸣�
��������1��207.1g��mol-1
��2���� ��A ��3���ܷ�Ӧ����ΪR4+����ǿ�����ԣ���Fe2+��ǿ��ԭ�ԣ�Pb4+��2Fe2+=pb2+��2Fe3+��4��Pb3O4��4HNO3=PbO2��2Pb��NO3��2��2H2O

��ϰ��ϵ�д�
�����Ŀ
 ����X�����о������������ڱ߳�Ϊ1.00��
����X�����о������������ڱ߳�Ϊ1.00�� ���������к���20��ԭ�ӣ�R�ڻ�ѧ��Ӧ�г�����Ϊ��2�ۡ���4�ۣ�����Һ��
���������к���20��ԭ�ӣ�R�ڻ�ѧ��Ӧ�г�����Ϊ��2�ۡ���4�ۣ�����Һ�� �ȶ�����
�ȶ����� ��ǿ�����ԣ��ɽ�NO����Ϊ
��ǿ�����ԣ��ɽ�NO����Ϊ ��������
��������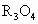 ����������
���������� ��Щ���ƣ�R����һ����������R��2��ͬλ�ؽ�Ϊ�ȶ����������γɺϽ��ڳ�ʪ���������γ����⣮R�ĵ��ʼ��������ڹ�ũҵ������ҽ����������ѧ�����ȸ��������кܶ���;��
��Щ���ƣ�R����һ����������R��2��ͬλ�ؽ�Ϊ�ȶ����������γɺϽ��ڳ�ʪ���������γ����⣮R�ĵ��ʼ��������ڹ�ũҵ������ҽ����������ѧ�����ȸ��������кܶ���;�� ��Һ�У��Ƿ�ᷢ����Ӧ��
��Һ�У��Ƿ�ᷢ����Ӧ�� ��Ӧ������R�������R�������κ�ˮ�������ʣ��˷�Ӧ�ķ���ʽΪ________��
��Ӧ������R�������R�������κ�ˮ�������ʣ��˷�Ӧ�ķ���ʽΪ________��