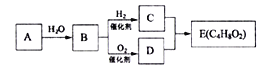
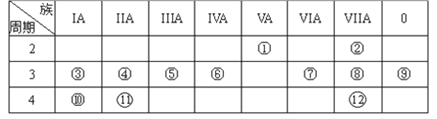
��Ŀ����
����Ŀ��A��B��C��D��E��F��G���ֶ���������Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㡣DԪ��ԭ�ӵ�����������Ϊm������������Ϊn��EԪ��ԭ�ӵ�L���ϵ�����Ϊ(m��n)��M���ϵ�����Ϊ![]() ��F��Dͬ���塣��ش��������⣺
��F��Dͬ���塣��ش��������⣺
(1)BԪ�������ڱ��е�λ����________��
(2)C��E�γɵĻ�����E3C����________(�ԭ�ӡ������ӡ����ӡ�)���塣
(3)��D���Ӻ�E���ӵİ뾶�ɴ�С____________�������ӷ��ţ�
(4)B��D����̬�⻯����ȶ���; _______��_______(�ѧʽ��
(5)F��һ����������������������Ϊ50����д������������G����ˮ��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
(6)��֪���ף�H2O�����������÷�Ӧ����������ԭ��Ӧ����������N��ClԪ����ɵĻ��������ӽṹģ������ͼ��ʾ��������Ư���ԡ��������Ԫ�صĻ��ϼ���________������H2O����ͬ�ĵ����������ĽṹʽΪ________��
![]()
���𰸡� ��2����IVA�� ���� O2- >Na+ CH4 H2O SO2 + Cl2 +2 H2O![]() H2SO4 + 2HCl ��1
H2SO4 + 2HCl ��1 ![]()
��������A��B��C��D��E��F��G���ֶ���������Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2������BΪ̼Ԫ�ء�A��һ��ԭ���У���������������֮��Ϊ�㣬��A������Ϊ��Ԫ�ء�DԪ��ԭ�ӵ�����������Ϊm������������Ϊn��EԪ��ԭ�ӵ�L���ϵ�����Ϊ(m��n)��M���ϵ�����Ϊ![]() ����n =2��m =6��DΪ��Ԫ�أ�CΪ
����n =2��m =6��DΪ��Ԫ�أ�CΪ![]() =1��EΪ��Ԫ�ء�F��Dͬ���壬��FΪ��Ԫ�أ���GΪ��Ԫ�ء�(1)BΪ̼Ԫ�أ������ڱ��е�λ���ǵ�2����IVA�壻(2)C��E�γɵĻ�����Na3N�������Ӻ�N3-���ӹ��ɣ��������Ӿ��壻(3)������ͬ���Ӳ�ṹ�����ӣ��˵����Խ��뾶ԽС���ʼ�D���Ӻ�E���ӵİ뾶�ɴ�СO2- >Na+��(4)Ԫ�طǽ�����Խǿ��̬�⻯����ȶ���Խǿ��B��D����̬�⻯����ȶ���; CH4��H2O��(5)F��һ����������������������Ϊ50������������Ϊ������������G����������ˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ��SO2 + Cl2 +2H2O
=1��EΪ��Ԫ�ء�F��Dͬ���壬��FΪ��Ԫ�أ���GΪ��Ԫ�ء�(1)BΪ̼Ԫ�أ������ڱ��е�λ���ǵ�2����IVA�壻(2)C��E�γɵĻ�����Na3N�������Ӻ�N3-���ӹ��ɣ��������Ӿ��壻(3)������ͬ���Ӳ�ṹ�����ӣ��˵����Խ��뾶ԽС���ʼ�D���Ӻ�E���ӵİ뾶�ɴ�СO2- >Na+��(4)Ԫ�طǽ�����Խǿ��̬�⻯����ȶ���Խǿ��B��D����̬�⻯����ȶ���; CH4��H2O��(5)F��һ����������������������Ϊ50������������Ϊ������������G����������ˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ��SO2 + Cl2 +2H2O![]() H2SO4 + 2HCl�� (6)��֪���ף�H2O�����������÷�Ӧ����������ԭ��Ӧ����������N��ClԪ����ɵĻ��������ӽṹģ������ͼ��ʾ��ΪNCl3��������Ư����Ϊ�����ᡣ��ΪNCl3����Ԫ�صĻ��ϼ���+1�ۣ�����H2O����ͬ�ĵ�����������Ϊ��������ṹʽΪ
H2SO4 + 2HCl�� (6)��֪���ף�H2O�����������÷�Ӧ����������ԭ��Ӧ����������N��ClԪ����ɵĻ��������ӽṹģ������ͼ��ʾ��ΪNCl3��������Ư����Ϊ�����ᡣ��ΪNCl3����Ԫ�صĻ��ϼ���+1�ۣ�����H2O����ͬ�ĵ�����������Ϊ��������ṹʽΪ![]() ��
��

����Ŀ���Ҷ���(�е�:197.3��)��һ����Ҫ�Ļ�������ԭ�ϡ���ú���ϳ���(��Ҫ�ɷ�CO��H2)���������Ʊ��õ����������(�е㣺164.5��)���ټ����Ӻϳ��Ҷ��������з�Ӧ�����º͡�������ȾС���ŵ㡣��Ӧ��������:
��ӦI:4NO(g)+4CH3OH(g)+O2(g)![]() 4CH3ONO(g)+2H2O(g) ��H1 =a kJ��mol-1
4CH3ONO(g)+2H2O(g) ��H1 =a kJ��mol-1
��ӦII:2CO(g)+2CH3ONO(g)![]() CH3OOCCOOCH3(l)+2NO(g) ��H2=b kJ��mol-1
CH3OOCCOOCH3(l)+2NO(g) ��H2=b kJ��mol-1
��ӦIII:CH3OOCCOOCH3(1)+4H2(g)![]() HOCH2CH2OH(1)+2CH3OH(g)��H3 =c kJ��mol-1
HOCH2CH2OH(1)+2CH3OH(g)��H3 =c kJ��mol-1
��ش���������:
(1)ú���ϳ�����Ӻϳ��Ҷ��������Ȼ�ѧ����ʽ��_____________________________����֪�÷�Ӧ�ڽϵ������������Է����С�˵���÷�Ӧ�ġ�H ______0(�>����<����=��)��
(2)CO��CH3ONO��0.4mol�ں��¡��ݻ��㶨Ϊ2L���ܱ������з�����ӦII���ﵽƽ��ʱCO�����������NO�����������ȣ�����÷�Ӧ�Ļ�ѧƽ�ⳣ��K=_____________������ʱ����������ͨ��0.4molNO,һ��ʱ��ﵽ��ƽ��ʱNO�����������ԭƽ��ʱ���______(�������ȡ�����С������ȷ����)��
(3)�¶ȸı�Է�ӦII�Ĵ���������Ӱ�죬���۴����Ļ��Բ���������ʱ���ʺ�CO��ѡ���Կɱ�ʾ����:
��ʱ����=CH3OOCCOOCH3����/��Ӧʱ������������
CO��ѡ����=�ϳ�[CH3OOCCOOCH3�����ĵ�CO�����ʵ���/��Ӧ����CO�����ʵ���]��100%
�ڲ�ͬ�¶��£�ijѧϰС�������������������ͬ�ķ�Ӧ������о���������ͬʱ��th����ÿ�ʱ���ʡ�CO��ѡ�����������±���ʾ��
��Ӧ�¶�(��) | ��ʱ����(g��mL-1��h-1) | CO��ѡ����(%) |
130 | 0.70 | ��72.5 |
140 | 0.75 | ��71.0 |
150 | 0.71 | ��55.6 |
160 | 0.66 | ��63.3 |
����˵����ȷ����________(����ĸ����)��
A.�¶����ߣ���ʱ������������С��˵����H2>0
B.�¶����ߣ�����������������CO��ѡ��������
C.�ۺϿ��ǿ�ʱ���ʺ�CO��ѡ���ԣ���ҵ����CH3OOCCOOCH3ʱ��ѡ��140��Ч�����
D.130��ʱ��CO��ѡ������ߣ�˵��CO����CH3OOCCOOCH3��ת�������
(4)120�桢��ѹʱ��CH3OOCCOOCH3+4H2![]() HOCH2CH2OH+2CH3OH��Ӧ�����е������仯��ͼ��ʾ������180�桢��ѹʱ������������÷�Ӧ�����е������仯ͼ��________
HOCH2CH2OH+2CH3OH��Ӧ�����е������仯��ͼ��ʾ������180�桢��ѹʱ������������÷�Ӧ�����е������仯ͼ��________

(5)�о�֤ʵ���Ҷ��������������ڼ�����Һ���γ�ȼ�ϵ�أ������ĵ缫��Ӧʽ��________________��