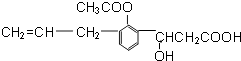
��Ŀ����
����Ŀ����һƿ�������Һ�����п��ܺ���NH4����K����Ba2����Al3����Fe3����I����NO3����CO32����SO42����AlO2����ȡ����Һ��������ʵ�飺
����pH��ֽ��⣬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ����
b����Һ��������
c��������ȫ�ܽ�
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ����
��ȡ�����۵õ��ļ�����Һ������Na2CO3��Һ���а�ɫ�������ɡ�
��������ʵ�����ش��������⡣
��1���ɢٿ����ų�__________�Ĵ��ڡ�
��2���ɢڿ���֤��__________�Ĵ��ڣ�ͬʱ�ų�______�Ĵ��ڣ�������_______________��
��3���ɢۿ���֤��________�Ĵ��ڣ�д��c��d���漰�Ļ�ѧ����ʽ�������ӷ�Ӧ�������ӷ���ʽ��ʾ�� c___________________________________________________ ��d___________________________________________________ ��
��4���ɢܿ����ų�__________�Ĵ��ڣ�ͬʱ֤��________�Ĵ��ڡ�
���𰸡� CO32����AlO2�� I�� Fe3����NO3�� CCl4����ֳ������ɫ��Fe3����NO3���ڸû����²�����I������ Al3����NH4�� Al(OH)3��OH��=AlO2����2H2O NH3��H2O ![]() NH3����H2O SO42�� Ba2��
NH3����H2O SO42�� Ba2��
����������1���ɢٿɵã���Һ�д��ڴ���H+��������Һ��һ��������CO32����AlO2����
��2����ΪI2��CCl4��Һ���Ϻ�ɫ�����ɢڿɵã�������ˮ�������I2��������Һ��һ����I������ΪFe3����NO3����������Һ�в�����I�����棬������Һ��һ��������Fe3����NO3����
��3����b��c�ɵã���ʼ�����ij���ֻ��Al(OH)3����ΪAl(OH)3�ܺ�NaOH��Һ��Ӧ���ɿ����Ե�NaAlO2������Һ��һ������Al3������d�ɵã���������Һ��������ΪNH3������Һ��һ������NH4�������Ϸ������ɢۿ���֤��Al3����NH4��һ�����ڣ�c��d���漰�ķ���ʽΪ��Al(OH)3��OH��=AlO2����2H2O��NH3��H2O ![]() NH3����H2O��
NH3����H2O��
��4���ɢܿɵã���ɫ����ΪBaCO3��������Һ��һ������Ba2������Ba2����SO42�����ܴ������棬�ʲ�����SO42����

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�����Ŀ��ijԭ��ص����ӷ���ʽ�� Zn + Cu2+ = Zn2+ + Cu����ԭ�����ȷ������� �� ��
���� | ���� | �������Һ | |
A�� | Cu | Zn | HCl |
B�� | Zn | Cu | CuSO4 |
C�� | Cu | Zn | CuSO4 |
D�� | Cu | Zn | ZnCl2 |
A. A B. B C. C D. D