��Ŀ����
(12��)��20 mL HCl��H2SO4�Ļ����Һ����μ���0.1 mol��L-1��Ba(OH)2��Һ�����ɳ�������������Һ��pH������Ba(OH)2��Һ����Ĺ�ϵ��ͼ5��ʾ��������������⣺
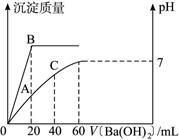
ͼ5
(1)��ʼʱ�����������������ʵ���Ũ��Ϊ______________ mol��L-1��
(2)��ʼʱ�����������������ʵ���Ũ��Ϊ______________ mol��L-1��
(3)B���ʾ�ij���������Ϊ______________g��
(4)A���ʾ��Һ�еģ�H+��Ϊ______________ mol��L-1��pHΪ______________��
(5)C���pHΪ______________(pH��ֱ���ö�����ʾ)��

ͼ5
(1)��ʼʱ�����������������ʵ���Ũ��Ϊ______________ mol��L-1��
(2)��ʼʱ�����������������ʵ���Ũ��Ϊ______________ mol��L-1��
(3)B���ʾ�ij���������Ϊ______________g��
(4)A���ʾ��Һ�еģ�H+��Ϊ______________ mol��L-1��pHΪ______________��
(5)C���pHΪ______________(pH��ֱ���ö�����ʾ)��
(1)0.1
(2)0.4
(3)0.466
(4)0.2 07
(5)1.18��-lg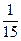
(2)0.4
(3)0.466
(4)0.2 07
(5)1.18��-lg
Ba2++ ====BaSO4��
====BaSO4��
H+ + OH-====H2O
1 1 1
n(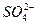 )=n��Ba(OH)2��="0.02" L��0.1 mol��L-1=2��10-3 mol
)=n��Ba(OH)2��="0.02" L��0.1 mol��L-1=2��10-3 mol
c(H2SO4)=n(H2SO4)/V(aq)=2��10-3 mol/002 L="0.1" mol��L-1
n(H+)=2��0.06 L��0.1 mol��L-1="0.012" mol
n(HCl)=n(H+)-2n(H2SO4)="0.012" mol-2��0.002 mol="0.008" mol
c(HCl)="n(HCl)/V(aq)=0.008" mol/0.02 L="0.4" mol��L-1
n(BaSO4)=n(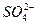 )="0.002" mol
)="0.002" mol
m(BaSO4)=n(BaSO4)��M(BaSO4)="0.002" mol��233 g��mol-1="0.466" g
(4)��A��ʱ��Ba(OH)2ǡ����H2SO4��ȫ��Ӧ����ʱ��Һ��ʣ���H+ȫ����HCl�ģ�n(H+)="n(HCl)=0.008" mol�����ԣ�c(H+)=n(H+)/V(��)="0.008" mol/0.04 L="0.2" mol��L-1,pH="-lg" 0.2=0.7��
(5)C��ʱ�Ļ��Һ����A��Ļ������ֵ���20 mL Ba(OH)2��Һ����ʱBa(OH)2ֻ��HCl��Ӧ��ʣ���H+��n(H+)="0.008" mol-2n��Ba(OH)2��="0.008" mol-2��0.1 mol��L-1��0.02 L="0.004" mol,c(H+)="0.004" mol/0.06 L="1/15" mol��L-1��pH=-lg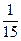 ��
��
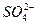 ====BaSO4��
====BaSO4��H+ + OH-====H2O
1 1 1
n(
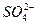 )=n��Ba(OH)2��="0.02" L��0.1 mol��L-1=2��10-3 mol
)=n��Ba(OH)2��="0.02" L��0.1 mol��L-1=2��10-3 mol c(H2SO4)=n(H2SO4)/V(aq)=2��10-3 mol/002 L="0.1" mol��L-1
n(H+)=2��0.06 L��0.1 mol��L-1="0.012" mol
n(HCl)=n(H+)-2n(H2SO4)="0.012" mol-2��0.002 mol="0.008" mol
c(HCl)="n(HCl)/V(aq)=0.008" mol/0.02 L="0.4" mol��L-1
n(BaSO4)=n(
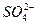 )="0.002" mol
)="0.002" molm(BaSO4)=n(BaSO4)��M(BaSO4)="0.002" mol��233 g��mol-1="0.466" g
(4)��A��ʱ��Ba(OH)2ǡ����H2SO4��ȫ��Ӧ����ʱ��Һ��ʣ���H+ȫ����HCl�ģ�n(H+)="n(HCl)=0.008" mol�����ԣ�c(H+)=n(H+)/V(��)="0.008" mol/0.04 L="0.2" mol��L-1,pH="-lg" 0.2=0.7��
(5)C��ʱ�Ļ��Һ����A��Ļ������ֵ���20 mL Ba(OH)2��Һ����ʱBa(OH)2ֻ��HCl��Ӧ��ʣ���H+��n(H+)="0.008" mol-2n��Ba(OH)2��="0.008" mol-2��0.1 mol��L-1��0.02 L="0.004" mol,c(H+)="0.004" mol/0.06 L="1/15" mol��L-1��pH=-lg
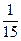 ��
��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
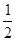 N2(g)+O2(g)====NO2(g) ��H��+34 kJ��mol-1���÷�ӦΪ_____________�������ȡ������ȡ�����Ӧ��
N2(g)+O2(g)====NO2(g) ��H��+34 kJ��mol-1���÷�ӦΪ_____________�������ȡ������ȡ�����Ӧ��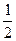 H2(g)+
H2(g)+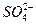 +Ba2++OH-====BaSO4��+H2O
+Ba2++OH-====BaSO4��+H2O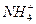 +OH-====NH3��+H2O
+OH-====NH3��+H2O