��Ŀ����
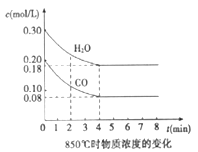
����Ŀ��������ʡ��������2017������ڶ���ģ�����ۻ�ѧ���⡿��1����һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO(g)+H2O(g)![]() CO2(g)+H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ����04 min��ƽ����Ӧ����v(CO)=______________ mol/(L��min)
CO2(g)+H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ����04 min��ƽ����Ӧ����v(CO)=______________ mol/(L��min)
��2��t1��(����850��)ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯���±���
ʱ��(min) | CO | H2O | CO2 | H2 |
0 | 0.200 | 0.300 | 0 | 0 |
2 | 0.138 | 0.238 | 0.062 | 0.062 |
3 | c1 | c2 | c3 | c4 |
4 | c1 | c2 | c3 | c4 |
5 | 0.116 | 0.216 | 0.084 | |
6 | 0.096 | 0.266 | 0.104 |
��ش�
������34 min֮�䷴Ӧ����_____________״̬��c3��ֵ_____________0.12 mol/L(����ڡ�С�ڻ����)��
����Ӧ��45 min�䣬ƽ�����淽���ƶ������ܵ�ԭ����_____________(��ѡ)������56 min֮����ֵ�����仯�����ܵ�ԭ����_____________(��ѡ)��
a��������ˮ�������� b�������¶� c��ʹ�ô��� d������ѹǿ
��3����ͼ��һ���绯ѧ��װ��ͼ��
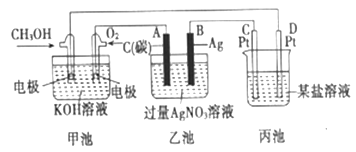
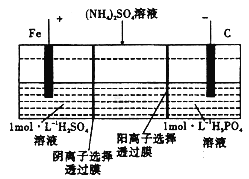
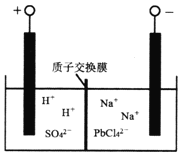
��ͼ���ҳ���________װ�ã�����ء���ԭ��ء�)�׳���OH-����____________��(�CH3OH����O2��)����������Ϊ400ml 1mol/L����ͭ��Һ�������·��1mol����ͨ��ʱ��C��D����������_______mol�����塣
��д��B�缫�ĵ缫��Ӧʽ��_______________________________________��
���𰸡� 0.03 ƽ�� С�� b a ���� CH3OH 0.35 Ag+��e-===Ag
��������(1)��(CO)= ![]() =
=![]() =0.03mol/(Lmin)���ʴ�Ϊ��0.03mol/(Lmin)��
=0.03mol/(Lmin)���ʴ�Ϊ��0.03mol/(Lmin)��
(2)���ڸ���850��ʱ������Ӧ����ѧ��Ӧ���ʼӿ죬һ����4minǰ�ﵽ��ѧƽ�⣮����ӱ��пɿ�����Ӧ��3min��4minʱ�ĸ�����Ũ����ͬ����3min-4min֮�䷴ӦӦ����ƽ��״̬�������Ƿ��ȷ�Ӧ���¶����ߣ���ѧƽ�����淴Ӧ�����ƶ���C3��ֵӦС��0.12 mol/L���ʴ�Ϊ��ƽ�⣻С�ڣ�
����Ӧ��4min-5min�䣬ƽ�����淽���ƶ������������¶ȡ�����������Ũ�ȡ����ٷ�Ӧ��Ũ�ȵ��������𣬹�ѡb������5min-6min֮��COŨ�ȼ��٣�H2OŨ������CO2Ũ������ֻ������ˮ������ʹ��ѧƽ��������Ӧ�����ƶ�����ѡa���ʴ�Ϊ��b��a��
(3)������ͼʾ��ͼ�м׳�����ȼ�ϵ�أ��ҳغͱ������ڵ��أ��׳���ͨ��״����Ǹ�����ͨ����������������ԭ��������������������OH-����CH3OH����������Ϊ400ml 1mol/L����ͭ��Һ��������ͭ0.4mol��0.4molͭ���ӷŵ�ת��0.8mol���ӣ������·��1mol����ͨ��ʱ�����������������ӷŵ�����0.25mol��������������0.2mol�����ӷŵ�����0.1mol����������������0.35mol�����壬�ʴ�Ϊ��������CH3OH��0.35��
���ҳ���B�缫Ϊ������������ԭ��Ӧ���缫��ӦʽΪAg+��e-=Ag���ʴ�Ϊ��Ag+��e-=Ag��