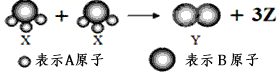
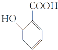
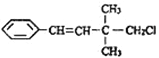
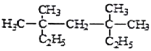��Ŀ����
����Ŀ��ʵ�����ù���Na2CO3����90mL 0.200 mol��L��1��Na2CO3��Һ���ش��������⣺
(1)ͨ������ó�������ƽ��ȡNa2CO3����________g��
(2)��Ҫʵʩ���ƣ�����ƽ���ձ����������⣬����IJ��������� _______ ��______��
(3)�ܽ�����У���������������__________________��
(4)������Ϻ�ʦָ������λͬѧ����������ijһ��������������Ϊ�������������лᵼ��������ҺŨ��ƫ�͵���____(��ѡ����ĸ)��
a.����ʱ��������ƿ�̶���
b.ת��ʱδϴ���ձ��Ͳ�����
c.����ƿʹ��ʱδ����
d.���ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶���
���𰸡�2.12 100mL����ƿ ��ͷ�ι� ���裬�����ܽ� bd
��������
(1)ѡ��ʹ��100mL������ƿ������c=![]() �������ʵ����ʵ�����Ȼ�����m=n��M����Na2CO3��������
�������ʵ����ʵ�����Ȼ�����m=n��M����Na2CO3��������
(2)�������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ȱ�ٵ�������
(3)���ݲ��������ܽ�ʱ�������ã�
(4)�����������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)�ù���Na2CO3����90mL0.200molL-1��Na2CO3��Һ��Ӧѡ��100mL����ƿ����Ҫ���ʵ�����m(Na2CO3)=0.2mol/L��0.1L��106g/mol=2.12g���ʺ�����Ϊ2.12��
(2)�������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪����������У�������ƽ��ҩ�ס��ձ�����������100mL����ƿ�ͽ�ͷ�ιܣ������������100mL����ƿ����ͷ�ιܣ�
(3)���������ܽ�ʱ�������ã����ٹ����ܽ⣻
(4)a.����ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�a���������⣻
b.��Һʱδϴ���ձ��Ͳ����������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�b�������⣻
c.����ƿʹ��ʱδ��������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬c���������⣻
d.���ݺ�����ƿ����ߨ�Ⱥ���Һ����ڿ̶��ߣ������˼���ˮ���̶��ߣ�������Һ���ƫ��ʹ��ҺŨ��ƫ�ͣ�d�������⣻
�ʺ���ѡ����bd��

 ������ϵ�д�
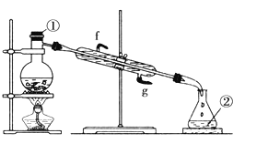
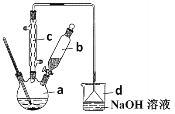
������ϵ�д�����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽װ��ͼ���й��������£�
��Ŀ | �� | �� | �屽 |
�ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
��1����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�塣����c��������____________�����ˮ��Ϊ_____���������������������ڣ�װ��d�е���©������Ϊ________��
��2��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������_______��
����ֳ��Ĵ��屽�м�����������ˮ����þ�����á����ˡ�
��3�������Ϸ��������Ҫ��һ���ᴿ�����в����б������____(������ȷѡ��ǰ����ĸ)��
A���ؽᾧ B������ C������ D����ȡ
��4����d�е�NaOH��Һ������������Һ������ֵ�����Ϊ_____________��������_____����������������������˵���÷�ӦΪȡ����Ӧ��
�����3��ʱ�õ�����е���Щ��Ϣ_______