��Ŀ����
�����ڹ�ũҵ���Ź㷺����;����֪25%��ˮ���ܶ�Ϊ0.91g/cm3��5%��ˮ���ܶ�Ϊ0.98g/cm3��
��1������100mL 2.5mol/L��ˮ��ҪŨ��Ϊ25%��ˮ
��2��������������Һ�������ϣ����ð�ˮ��Һ������������
A������15% B������15% C��С��15% D�������㣮
����֪��4NH3+5O2
4NO+6H2O��4NO+3O2+2H2O��4HNO3
��1����������������������Ϊ0.20���������������Ϊ0.80��
��a mol NO��ȫת��ΪHNO3��������Ҫ����
��ΪʹNH3ǡ����ȫ����ΪNO����-������������а��������������С����ʾ��Ϊ
��2��20.0mol NH3�ÿ����������������������Ϊ��NO 18.0mol��O2 12.0mol��N2 150.0mol��һ�������ᣬ�Լ������ɷ֣�������NO��O2�����ϣ������㰱ת��ΪNO��HNO3��ת���ʣ�
��3��20.0mol NH3��һ����������ַ�Ӧ����ת��Ϊ���ᣮͨ�����㣬��ͼ�л���HNO3�����ʵ���n��A���Ϳ��������ʵ���n ��B����ϵ���������ߣ�

��1������100mL 2.5mol/L��ˮ��ҪŨ��Ϊ25%��ˮ
18.68
18.68
mL������2λС��������2��������������Һ�������ϣ����ð�ˮ��Һ������������
C
C
��A������15% B������15% C��С��15% D�������㣮
����֪��4NH3+5O2
| ���� | �� |
��1����������������������Ϊ0.20���������������Ϊ0.80��
��a mol NO��ȫת��ΪHNO3��������Ҫ����
0.75a
0.75a
mol����ΪʹNH3ǡ����ȫ����ΪNO����-������������а��������������С����ʾ��Ϊ
0.14
0.14
������2λС��������2��20.0mol NH3�ÿ����������������������Ϊ��NO 18.0mol��O2 12.0mol��N2 150.0mol��һ�������ᣬ�Լ������ɷ֣�������NO��O2�����ϣ������㰱ת��ΪNO��HNO3��ת���ʣ�
��3��20.0mol NH3��һ����������ַ�Ӧ����ת��Ϊ���ᣮͨ�����㣬��ͼ�л���HNO3�����ʵ���n��A���Ϳ��������ʵ���n ��B����ϵ���������ߣ�

������I��1���ȸ���C=
����Ũ��ˮ�����ʵ���Ũ�ȣ��ٸ�����Һϡ��ǰ�����ʵ����ʵ����������Ũ��ˮ�������
��2����ˮŨ��Խ���ܶ�ԽС�����������ʱ��Ũ�Ƚ�ϡ�İ�ˮ�����ϴ���Ũ�İ�ˮ��������С��������Ϻ�������ҺŨ�ȵ�ȻҪ��15%ƫСЩ��
II��1���ٸ���һ������������֮��Ĺ�ϵʽ���㣻
�ڸ��ݰ���������Ҫ�����������ٸ������������ʽ���м��㣻
��2�����ݷ�Ӧ�����е�������������м��㣬���������������ʵ������ٸ���ԭ���غ����ת���ʣ�
��3�����ü����ҳ�����������������Ĺ�ϵ������������Ϳ����Ĺ�ϵͼ��
| 1000��w |
| M |
��2����ˮŨ��Խ���ܶ�ԽС�����������ʱ��Ũ�Ƚ�ϡ�İ�ˮ�����ϴ���Ũ�İ�ˮ��������С��������Ϻ�������ҺŨ�ȵ�ȻҪ��15%ƫСЩ��
II��1���ٸ���һ������������֮��Ĺ�ϵʽ���㣻
�ڸ��ݰ���������Ҫ�����������ٸ������������ʽ���м��㣻
��2�����ݷ�Ӧ�����е�������������м��㣬���������������ʵ������ٸ���ԭ���غ����ת���ʣ�
��3�����ü����ҳ�����������������Ĺ�ϵ������������Ϳ����Ĺ�ϵͼ��
����⣺��1��Ũ��ˮ�����ʵ���Ũ��=
mol/L=13.4mol/L����Ũ��ˮ�����Ϊv��2.5mol/L��0.1L=13.4mol/L��V��V=0.01868L=18.68mL��
�ʴ�Ϊ��18.68mL��
��2�������ְ�ˮ��������ϣ����Ϻ�ˮ����������Ϊ15%������������ְ�ˮ��Ũ���ܶȽ�С������������С�����ְ�ˮ��Ϻ������������ӽ�ϡ��ˮ��Ũ�ȣ����ð�ˮ��Һ����������С��15%����ѡC��
��2���ٸ���4NO+3O2+2H2O��4HNO3֪��a mol NO��ȫת��ΪHNO3��������Ҫ����=
��3=0.75amol���ʴ�Ϊ��0.75a��
�ڼ��谱���������4L������Ҫ���������Ϊy��
4NH3+5O2
4NO+6H2O
4 5
4L 0.20y
y=
L=25L��
��-�������������������=
=0.14��
�ʴ�Ϊ��0.14��
��2��������x mol HNO3���������������������4����4��2x+18.0��
+12.0��=150.0 x=1.5��mol��
���ݵ�ԭ���غ㣬NH3��ת��ΪHNO3��ת����=
��100%=7.5%
NH3��ת��ΪNO��ת����=
��100%=97.5%��
�𣺰�ת��ΪNO��HNO3��ת���ʷֱ���7.5%��97.5%��
��3��4NH3+5O2
4NO+6H2O�٣�4NO+3O2 +2H2O��4HNO3�ڣ���
��NH3+2O2��H2O+HNO3�ۣ��ɢ٢�֪����n��O2����n��NH3����5��4����n����������n��NH3����25��4ʱ���������ɣ���ʱ20.0mol�������������Ϊ��
��20.0mol=125mol��
��5��4��n��O2����n��NH3����2��1����25��4��n����������n��NH3����10��1ʱ���������ɣ���ʱ20.0mol�������������Ϊ10��20.0mol=200mol��������������ʵ�����20mol������HNO3�����ʵ���n��A���Ϳ��������ʵ���n ��B����ϵ����������Ϊ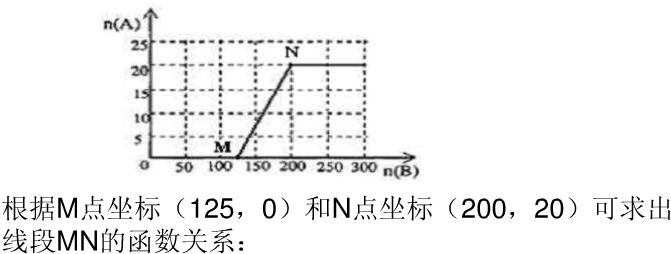 ��
��
�ʴ�Ϊ�� ��
��
| 1000��0.91��25% |
| 17 |
�ʴ�Ϊ��18.68mL��
��2�������ְ�ˮ��������ϣ����Ϻ�ˮ����������Ϊ15%������������ְ�ˮ��Ũ���ܶȽ�С������������С�����ְ�ˮ��Ϻ������������ӽ�ϡ��ˮ��Ũ�ȣ����ð�ˮ��Һ����������С��15%����ѡC��
��2���ٸ���4NO+3O2+2H2O��4HNO3֪��a mol NO��ȫת��ΪHNO3��������Ҫ����=
| amol |
| 4 |
�ڼ��谱���������4L������Ҫ���������Ϊy��
4NH3+5O2
| һ������ |
4 5
4L 0.20y
y=
| 4L��5 |
| 4��0.20 |
��-�������������������=
| 4L |
| 4L+25L |
�ʴ�Ϊ��0.14��
��2��������x mol HNO3���������������������4����4��2x+18.0��
| 5 |
| 4 |
���ݵ�ԭ���غ㣬NH3��ת��ΪHNO3��ת����=
| 1.5mol |
| 20.0mol |
NH3��ת��ΪNO��ת����=
| 18.0+1.5 |
| 20 |
�𣺰�ת��ΪNO��HNO3��ת���ʷֱ���7.5%��97.5%��
��3��4NH3+5O2
| ���� |
| �� |
| ��+�� |
| 2 |
| 5 |
| 4 |
��5��4��n��O2����n��NH3����2��1����25��4��n����������n��NH3����10��1ʱ���������ɣ���ʱ20.0mol�������������Ϊ10��20.0mol=200mol��������������ʵ�����20mol������HNO3�����ʵ���n��A���Ϳ��������ʵ���n ��B����ϵ����������Ϊ
 ��
���ʴ�Ϊ��
 ��
�����������⿼�鰱�������ʺ��йط�Ӧ���ѵ��ǻ�������Ϳ������ʵ���֮��Ĺ�ϵͼ���ü�������������ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 4NO+6 H2O��4NO+3O2+2H2O
4NO+6 H2O��4NO+3O2+2H2O 4HNO3
4HNO3
 4NO+6NO��4NO+3O2+2H2O
4NO+6NO��4NO+3O2+2H2O 4HNO3
4HNO3
 4NO+6H2O��4NO+3O2+2H2O��4HNO3
4NO+6H2O��4NO+3O2+2H2O��4HNO3
 4NO+6H2O��4NO+3O2+2H2O��4HNO3
4NO+6H2O��4NO+3O2+2H2O��4HNO3