��Ŀ����
������ѧ��ѧ����Ԫ��ԭ�ӽṹ���������±���ʾ��
��1��AԪ�������ڱ��е�λ��Ϊ_______________________________________________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ_______________________________________��������________(����ӡ����ۡ�)�����
��3����F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����__________________________________________��
��F��C��ɵ����ֻ�����M��N�����ĵ������ֱ���X��Y��ȣ���M��ˮ��Һ��________________�ԣ�N�ĽṹʽΪ______________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ����________________________________________________________��
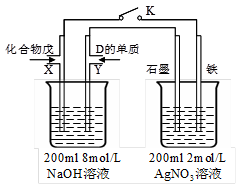
��5��������ΪB��D�ĵ����õ������Ӻ����NaOH��Һ�п����γ�ԭ��أ�����Ϊ�Ƿ���ԣ������ԣ���д�������ĵ缫����ʽ(����Ϊ���пɲ�д)��______________________________________________________________��
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�����������еij������������������Ȼ����Է����������35.5 |
| �� | B | Bԭ���������������ڲ����������1/5 |
| �� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
| �� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
| �� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
| �� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
��1��AԪ�������ڱ��е�λ��Ϊ_______________________________________________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ_______________________________________��������________(����ӡ����ۡ�)�����
��3����F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����__________________________________________��
��F��C��ɵ����ֻ�����M��N�����ĵ������ֱ���X��Y��ȣ���M��ˮ��Һ��________________�ԣ�N�ĽṹʽΪ______________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ����________________________________________________________��
��5��������ΪB��D�ĵ����õ������Ӻ����NaOH��Һ�п����γ�ԭ��أ�����Ϊ�Ƿ���ԣ������ԣ���д�������ĵ缫����ʽ(����Ϊ���пɲ�д)��______________________________________________________________��
��1���������ڵڢ���
��2��Mg3N2������
��3���ٷֱ�ȡX��Y�����������Թ��У��ٸ�����������MnO2��ĩ��Ѹ�ٲ�����ɫ�������H2O2���������������H2O(��������)
�ڼHNHNHH
��4��4NH3��3O2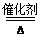 2N2��6H2O(��������)
2N2��6H2O(��������)
��5��Si��4e����6OH��=SiO32-��3H2O
��2��Mg3N2������
��3���ٷֱ�ȡX��Y�����������Թ��У��ٸ�����������MnO2��ĩ��Ѹ�ٲ�����ɫ�������H2O2���������������H2O(��������)
�ڼHNHNHH
��4��4NH3��3O2
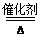 2N2��6H2O(��������)
2N2��6H2O(��������)��5��Si��4e����6OH��=SiO32-��3H2O
���ݱ������ṩ����Ϣ�������ƶ�AΪFe��BΪMg��CΪN��DΪSi��EΪO��FΪH��
��1��Feλ�ڵ������ڵڢ��塣
��2��Mg��N�γɵĻ�����ΪMg3N2���������ӻ����
��3���ٿ�������MnO2�ܹ����ֽ�H2O2������������H2O��H2O2����H2O��H2O2�ĵ������ֱ�Ϊ10��18��N��H�γɵ�10�����Ӻ�18�����ӵĻ�����ֱ�ΪNH3��N2H4������N2H4�ĽṹʽΪHNHNHH��
��4��O2��������ǿ��N2������ͨ�����ʵ��û���Ӧ��֤��
��5��Mg��Si�ĵ����õ������Ӳ���NaOH��Һ�п����γ�ԭ��أ�Si���������缫��ӦʽΪSi��4e����6OH��=SiO32-��3H2O��
��1��Feλ�ڵ������ڵڢ��塣
��2��Mg��N�γɵĻ�����ΪMg3N2���������ӻ����
��3���ٿ�������MnO2�ܹ����ֽ�H2O2������������H2O��H2O2����H2O��H2O2�ĵ������ֱ�Ϊ10��18��N��H�γɵ�10�����Ӻ�18�����ӵĻ�����ֱ�ΪNH3��N2H4������N2H4�ĽṹʽΪHNHNHH��
��4��O2��������ǿ��N2������ͨ�����ʵ��û���Ӧ��֤��
��5��Mg��Si�ĵ����õ������Ӳ���NaOH��Һ�п����γ�ԭ��أ�Si���������缫��ӦʽΪSi��4e����6OH��=SiO32-��3H2O��

��ϰ��ϵ�д�
�����Ŀ
 ,1��
,1�� �������
�������