��Ŀ����
(10��)�û���Ӧ��ͨʽ�ɱ�ʾΪ(ת�����漰�ľ�Ϊ��ѧ��ѧ�ij�������):
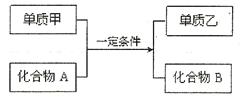
��ش��������⣺
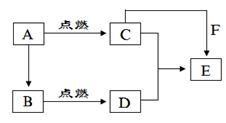
(1)�����ʼ��뻯����A�����ķ�Ӧ������Ұ�⺸�Ӹֹ죬��÷�Ӧ�Ļ�ѧ����ʽΪ ��
(2)���ס��ҷֱ���ͬ��������ַǽ������嵥�ʣ�����A��һ����Ҫ��;�� ��
(3)���ס��ҷֱ���ͬ���������Ԫ�ص����ֵ��ʣ�������B��һ��ǿ���Ӧ�����ӷ���ʽΪ ��
(4)����ɵ����ҵ�Ԫ�ص������������Ǵ�����������2�����������ʼ����ڻ�����A��ȼ�գ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ ������Ӧ������2.4g�����ң������ĵĻ�����A�ڱ�״���µ����Ϊ ��

��ش��������⣺
(1)�����ʼ��뻯����A�����ķ�Ӧ������Ұ�⺸�Ӹֹ죬��÷�Ӧ�Ļ�ѧ����ʽΪ ��
(2)���ס��ҷֱ���ͬ��������ַǽ������嵥�ʣ�����A��һ����Ҫ��;�� ��
(3)���ס��ҷֱ���ͬ���������Ԫ�ص����ֵ��ʣ�������B��һ��ǿ���Ӧ�����ӷ���ʽΪ ��
(4)����ɵ����ҵ�Ԫ�ص������������Ǵ�����������2�����������ʼ����ڻ�����A��ȼ�գ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ ������Ӧ������2.4g�����ң������ĵĻ�����A�ڱ�״���µ����Ϊ ��
����10�֣���1��2Al+Fe2O3 Al2O3+2Fe(2��) (2)�����ά��2�֣�
Al2O3+2Fe(2��) (2)�����ά��2�֣�
��3��2Na+2H2O=2Na++2OH-+H2��(2��) (4)1:2(2��) 4.48L��2�֣�
 Al2O3+2Fe(2��) (2)�����ά��2�֣�
Al2O3+2Fe(2��) (2)�����ά��2�֣���3��2Na+2H2O=2Na++2OH-+H2��(2��) (4)1:2(2��) 4.48L��2�֣�
(1)�����ʼ��뻯����A�����ķ�Ӧ������Ұ�⺸�Ӹֹ죬��Ӧһ�������ȷ�Ӧ������ʽ��2Al+Fe2O3 Al2O3+2Fe��
Al2O3+2Fe��
(2)���ס��ҷֱ���ͬ��������ַǽ������嵥�ʣ�����ҷֱ���̼�裬��A�Ƕ������裬B��CO�������������Ҫ��;֮һ�������ά��
(3)���ס��ҷֱ���ͬ���������Ԫ�ص����ֵ��ʣ�������B��һ��ǿ���B���������ƣ�A��ˮ�������ơ�������������Ӧ�ķ���ʽ��2Na+2H2O=2Na++2OH-+H2����
��4��Ԫ�ص������������Ǵ�����������2����������̼���������ʼ����ڻ�����A��ȼ�գ�����A��CO2������þ��B������þ������̼����ҵ�ķ���ʽˮ��2Mg��CO2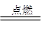 2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��
2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��
 Al2O3+2Fe��
Al2O3+2Fe��(2)���ס��ҷֱ���ͬ��������ַǽ������嵥�ʣ�����ҷֱ���̼�裬��A�Ƕ������裬B��CO�������������Ҫ��;֮һ�������ά��
(3)���ס��ҷֱ���ͬ���������Ԫ�ص����ֵ��ʣ�������B��һ��ǿ���B���������ƣ�A��ˮ�������ơ�������������Ӧ�ķ���ʽ��2Na+2H2O=2Na++2OH-+H2����
��4��Ԫ�ص������������Ǵ�����������2����������̼���������ʼ����ڻ�����A��ȼ�գ�����A��CO2������þ��B������þ������̼����ҵ�ķ���ʽˮ��2Mg��CO2
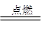 2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��
2MgO��C������CO2����������þ�ǻ�ԭ�������ߵ����ʵ���֮����1:2��2.4̼��0.2mol��������ҪCO2��0.2mol����״���µ�������4.48L��
��ϰ��ϵ�д�
�����Ŀ