��Ŀ����
���ȵ�ϡ������Һ���ܽ���11.4gFeSO4��������50mL0.5mo1��L��1KNO3��Һ��ʹ���е�Fe2��ȫ��������Fe3����KNO3Ҳ��Ӧ��ȫ������NxOy�������������ݳ�����FeSO4����KNO3����H2SO4����k2SO4����Fe2��SO4��3����NxOy����H2O
��1�������x��________��y��________��
��2����ƽ������ѧ����ʽ���������ת�Ƶķ������Ŀ��
��3����Ӧ�е���������________��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
 ��2007?�Ͳ���ģ������������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᣮij��ѧ�о�С����ʵ�����а����в���ģ����������̣���д���пհף�
��2007?�Ͳ���ģ������������������һ�ֳ��õĽ������洦������������ʹ���ı�������һ�����ܵ�����Ĥ��������Ĥ������ϡ���ᣮij��ѧ�о�С����ʵ�����а����в���ģ����������̣���д���пհף� �Խ���������д������γ�һ�����ܵ������ﱣ��Ĥ���Ƿdz���Ч��һ�ֽ�������������
�Խ���������д������γ�һ�����ܵ������ﱣ��Ĥ���Ƿdz���Ч��һ�ֽ�������������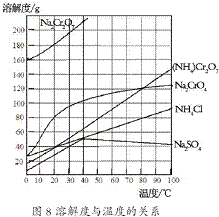 ��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�
��2012?����һģ���ظ����[��NH4��2Cr2O7]��һ�ֽۻ�ɫ�ᾧ���������л��ϳɴ�����ʵ�����ƴ�����N2��Cr2O3�ȣ�ʵ���ҿ��ɹ�ҵ�������ƣ�Na2CrO4��Ϊԭ����ȡ���й������ܽ����ͼ��ʾ��ʵ�鲽�����£�