��Ŀ����
��֪��Ӧ��

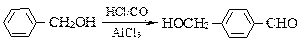 ��
��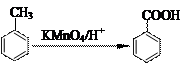
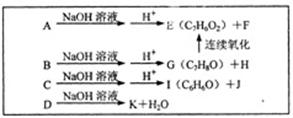
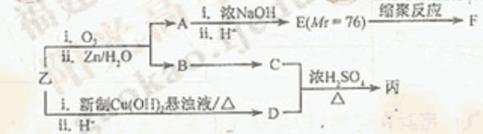
 ��������A~I��ת����ϵ����ͼ��
��������A~I��ת����ϵ����ͼ��

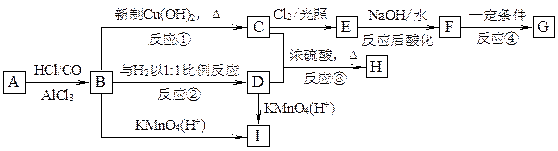
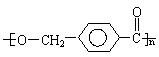
 ��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺
��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺
 (1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� ��
(1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� ��
 (2)д���������ʵĽṹ��ʽ��F ��I ��A ��
(2)д���������ʵĽṹ��ʽ��F ��I ��A ��
 (3)д�����з�Ӧ�Ļ�ѧ����ʽ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
 ��B��C�� ��
��B��C�� ��
 ��C+D��H�� ��
��C+D��H�� ��
 ��F��G�� ��
��F��G�� ��
 (4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��
(4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��

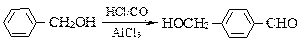 ��
��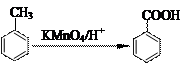
 ��������A~I��ת����ϵ����ͼ��
��������A~I��ת����ϵ����ͼ��
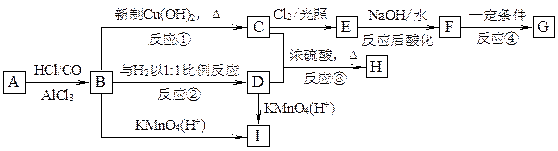
 ��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺
��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺ (1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� ��
(1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� �� (2)д���������ʵĽṹ��ʽ��F ��I ��A ��
(2)д���������ʵĽṹ��ʽ��F ��I ��A �� (3)д�����з�Ӧ�Ļ�ѧ����ʽ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ�� ��B��C�� ��
��B��C�� �� ��C+D��H�� ��
��C+D��H�� �� ��F��G�� ��
��F��G�� �� (4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��
(4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��(1)���ۣ�������(2) HOCH2�� ��COOH��HOOC��
��COOH��HOOC�� ��COOH��
��COOH�� ��CH3��
��CH3��
 (3)��H3C��
(3)��H3C�� ��CHO + 2Cu(OH)2
��CHO + 2Cu(OH)2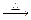 H3C��
H3C�� ��COOH + Cu2O��+ 2H2O��
��COOH + Cu2O��+ 2H2O��

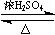 ��H3C��
��H3C�� ��COOH+HOCH2��
��COOH+HOCH2�� ��CH3 H3C��
��CH3 H3C�� ��COOCH2��
��COOCH2�� ��CH3+H2O
��CH3+H2O

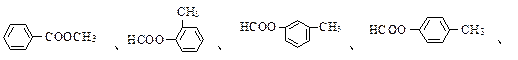
 ��nHOCH2��
��nHOCH2�� ��COOH + nH2O
��COOH + nH2O
 (4)6��
(4)6��


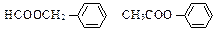 ����дһ�֡�
����дһ�֡�
 ��COOH��HOOC��
��COOH��HOOC�� ��COOH��
��COOH�� ��CH3��
��CH3�� (3)��H3C��
(3)��H3C�� ��CHO + 2Cu(OH)2
��CHO + 2Cu(OH)2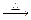 H3C��
H3C�� ��COOH + Cu2O��+ 2H2O��
��COOH + Cu2O��+ 2H2O��
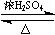 ��H3C��
��H3C�� ��COOH+HOCH2��
��COOH+HOCH2�� ��CH3 H3C��
��CH3 H3C�� ��COOCH2��
��COOCH2�� ��CH3+H2O
��CH3+H2O

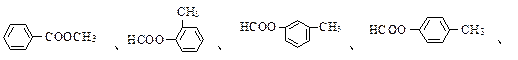
 ��nHOCH2��
��nHOCH2�� ��COOH + nH2O
��COOH + nH2O (4)6��
(4)6��

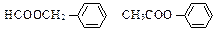 ����дһ�֡�
����дһ�֡���

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
�����Ŀ
 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��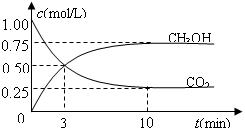
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ�����������������������������������������������������������������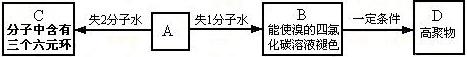

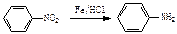
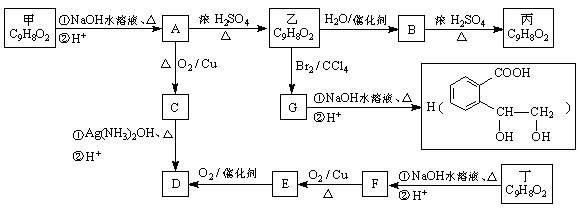
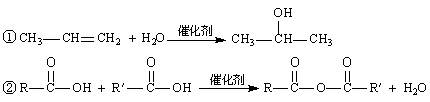

 �ṹ)����X�Ľṹ��ʽΪ (��дһ��)��
�ṹ)����X�Ľṹ��ʽΪ (��дһ��)��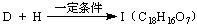 �������������I���� ��
�������������I���� �� ��˵���У���ȷ����
��˵���У���ȷ����
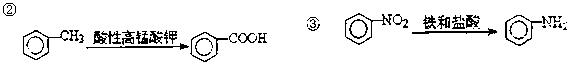

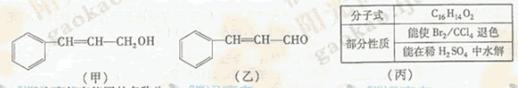
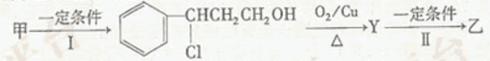

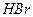 c��
c�� 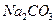 ��Һ d�� ����
��Һ d�� ����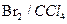 �����ӳɷ�Ӧ d����
�����ӳɷ�Ӧ d���� ��Һ��ʾ������ɫ
��Һ��ʾ������ɫ