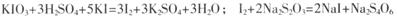��Ŀ����
(12��)ijʵ��С����������ͼ��ʾװ������й�ʵ�顣

(1) �й��������ȡ���ռ���β����������
������a�����ƣ�__________����װ��I������ȡSO2����Ӧ�Ļ�ѧ����ʽΪ: __________
װ��III��������������գ�����������Cl2,��IH����ʢ��Һ��Ϊ____________________
A.ˮ B.Ũ���� C. NaOH��Һ D.����NaCl��Һ
��װ��II�������ռ�װ��Ҳ������ϴ��װ��.�������ռ�N0������������������____________________
(2) �Ƚ�̼��������Ԫ�صķǽ�����ǿ����a��װ�����ᡢװ��II��װ��Na2SiO3 ��Һ��
��װ��II��ʢ�ŵ���ҺΪ____________________
װ��III�з�Ӧ�����ӷ���ʽΪ____________________
���𰸡�
����������

��ϰ��ϵ�д�
�����Ŀ
�����12�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������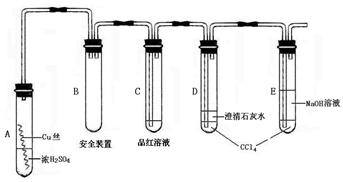
ʵ��ѡ��ϸͭ˿��98.3% H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
|

�����������ϻش���������
��1��A�Թ��Ϸ��ij����ܵ�������_________________��D��E��֧�Թ���CCl4��������_____________��
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��____��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ����ǣ�___________�����ʵ����֤��IJ���________________��
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���_____����д��ѧʽ��