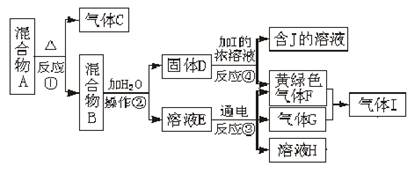
��Ŀ����
�����й�ʵ��ԭ���������ͽ��۶���ȷ���ǣ�
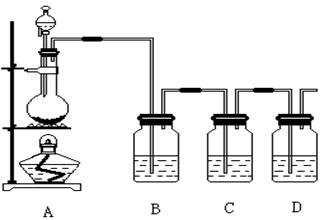
| A��ij��ɫ��Һ�μ���ˮ��CCl4�������÷ֲ���²���Һ����ɫ����ԭ��Һ����I�� |
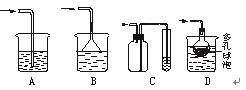
| B��ȡ������ҺX�������м����������ư�ˮ���ټӼ���KSCN��Һ����Һ��죬 ˵��X��Һ��һ������Fe2�� |
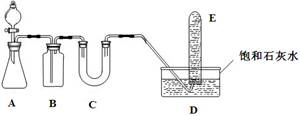
| C����ij��Һ�еμ�BaCl2��Һ�����а�ɫ�������ٵμ�����ϡHNO3�����������ܽ⣬ ��˵��ԭ��Һ��һ����SO42�� |
| D��ij��ɫ��Һ�ýྻ��˿պȡ��Һ������ɫ��Ӧ������ʻ�ɫ����ԭ��Һ����Na+��K+ |
A
A����ȡ���̣���ȷ��
B������ԭ��Һ����Fe3+��
C���������ǿ�����ԣ�ԭ��Һ��������������ܱ�����Ϊ�������
D����Ҫ�������ܲ������ܿ����Ƚ����Ե���ɫ��
B������ԭ��Һ����Fe3+��
C���������ǿ�����ԣ�ԭ��Һ��������������ܱ�����Ϊ�������
D����Ҫ�������ܲ������ܿ����Ƚ����Ե���ɫ��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ