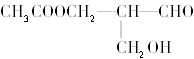��Ŀ����
����Ŀ��ʵ������Ҫ0.2 mol/L NaOH��Һ450 mL��0.5 mol/L������Һ500 mL��������������Һ����������ش��������⣺
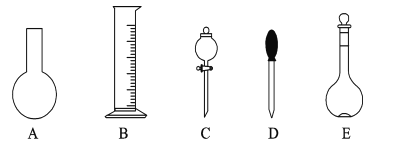
(l)��ͼ��ʾ��������������Һһ������Ҫ���� ___������ĸ��ţ�������������Һ�����õ��IJ���������____���������ƣ���
(2)������NaOH��Һʱ��
�ٸ��ݼ��㣬��������ƽ��ȡNaOH����____g��
����NaOH��Һ��ת��������ƿʱ�������������������õ���ҺŨ��_____�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
(3)������������Һʱ��
��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ____mL������������һλС������
�����ʵ������15 mL��20 mL��50 mL��Ͳ��Ӧѡ�� ___mL��Ͳ��á�
���𰸡�AC �ձ��������� 4.0 ƫ�� 13.6 15
��������
����һ�����ʵ���Ũ����Һ�����ƹ��̷�������Ҫ��ʵ���������������ʵ���Ũ�ȵļ��㹫ʽ���в��������е�ʵ����������
AΪƽ����ƿ��CΪ��Һ©������������Һ�����в���ʹ�õ���ƿ�ͷ�Һ©��������һ��Ũ�ȵ���Һ��ȱ�ٲ������ͽ�ͷ�ιܣ��ʴ�Ϊ��AC���ձ��������� ��
����0.2 mol/L NaOH��Һ450 mL����Ҫ500mL����ƿ���ʼ�����Ҫ��500mL����m(NaOH)=0.2![]() 0.5L
0.5L![]() g/mol=4.0g�����ݼ��㣬��������ƽ��ȡNaOH����4.0g����NaOH��Һ��ת��������ƿʱ�������������������ʵ����ʵ������٣����õ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��4.0��ƫ�ͣ�
g/mol=4.0g�����ݼ��㣬��������ƽ��ȡNaOH����4.0g����NaOH��Һ��ת��������ƿʱ�������������������ʵ����ʵ������٣����õ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��4.0��ƫ�ͣ�
��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��![]() =18.4mol/L��
=18.4mol/L��
����500��mL 0.5mol/L�����ᣬ��ҪŨ��������Ϊ��0.5mol/L��0.5L=18.4mol/L![]() V��V��0.0136L=13.6mL�����ݡ����������ԭ��ѡ��15mL��Ͳ���ʴ�Ϊ��13.6mL��15mL��
V��V��0.0136L=13.6mL�����ݡ����������ԭ��ѡ��15mL��Ͳ���ʴ�Ϊ��13.6mL��15mL��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ţ�̺ͽ�֭��ϣ���ţ�����̱��Ϊһ�ָ��й㶫��ɫ����Ʒ������ײ�̡�Ϊ������ţ�����������������ijͬѧ�ڲ�ͬ�¶ȵĵ���ţ���л���һЩ���ʽ�֭���۲�����15min�������Ƿ�����̣�������±�����ش��������⣺
�¶ȣ����� | 20 | 40 | 60 | 80 | 100 |
��� | 15min����δ�����̼��� | 14min����ȫ���� | 1min����ȫ���� | 1min����ȫ���� | 15min����δ�����̼��� |
��ע��������еĽ�֭�ظ�����ʵ�飬ţ�����κ��¶��¾��������̣�
��1��ʵ��֤�����ʽ�֭�к���һ��ø���������� ��
��2��20����100��ʱ��15min����δ�����̼���˵��ø�Ļ��Խϵͣ���ԭ��ֱ��� �� ��
��3����60��ʱţ�����н�֭��û�н�֭������¶��������̣�����Ӧ���е�tʱ�������м��˽�֭����ͼ������ȷ��ʾ�ӽ�֭��ţ��������ʱ��仯���Ƶ������� ��

��4��Ϊ���ʵ���ȷ�ȣ�ʵ��������ͬ�¶ȵĵ���ţ���л���һЩ���ʽ�֭��������Ӧע����� ��
��5����ͬѧ˵����ʵ�鲻�ܵó���֭ʹţ�����̵������¶ȣ�������������: ��
����Ŀ��H2Y2-���Ҷ����������(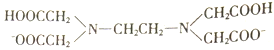 )�ļ�д��������ֽ��������γ�����
)�ļ�д��������ֽ��������γ�����
I. H2Y2-��Fe2+�γɵ������FeY2-���������������е�NO��������ԭ��:
FeY2-(aq)+NO(g)![]() FeY2-(NO)(aq) ��H<0
FeY2-(NO)(aq) ��H<0
��1������NO��������һ��������ͨ����ʼ�¶�Ϊ50����FeY2-��Һ�С�NO��������ͨ��������ʱ��仯����ͼ��ʱ��Խ����NO������Խ�͵�ԭ����_________��
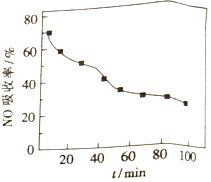
��2�����ɵ�FeY2-(NO)(aq)��ͨ���������ۻ�ԭ��������ԭ��:FeY2-(NO)(aq)+Fe+H2O��FeY2-(aq)+Fe(OH)2+NH3(δ��ƽ)��
������Һ��������14g���ۣ��������յ������к���NO�����ʵ���Ϊ_______��
II.���ͭ(CuY2-)��ˮ�Ĵ���һֱ�ǻ�������������о��ȵ㡣
��1��H2Y2-��Cu2+��Fe3+��Ca2+���������±�:
��Ϸ�Ӧ | lgK(KΪƽ�ⳣ��) |
Cu2++H2Y2- | 18.8 |
Fe3++H2Y2- | 25.1 |
Ca2++H2Y2- | 10.7 |
��������ȶ��Ľ����������_____(�ѧʽ)���������ͭ(CuY2-)��ˮ�м���һ�ֻ�ɫ������ҺA�ɽ����Cu2+������A��������Ϊ________(�ѧʽ)��
������pH�ɽ��������Cu2+ת��ΪCu(OH)2��������Ҫʹc(Cu2+)��2.2��10-4mol/L��pHӦ������________(������Ksp[Cu(OH)2]= 2.2��10-20)��
��2���ǻ����ɻ�(-OH)��Na2FeO2�����������ͭ�е�Y4-��ʹCu2+�õ����롣
��������������-OH�ɽ�Y4-(C10H12O8N24-)��������CO2��H2O��N2���÷�Ӧ�����ӷ���ʽΪ___________________��
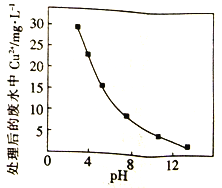
��Na2FeO4�����������²��ȶ�����Na2FeO4������ķ�ˮ��Cu2+��Ũ����pH�Ĺ�ϵ����ͼ��pHԽ����/span>��ˮ����Ч��Խ�ã�������Ϊ______________��