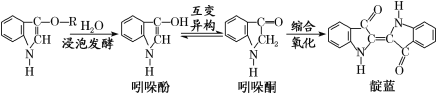
��Ŀ����
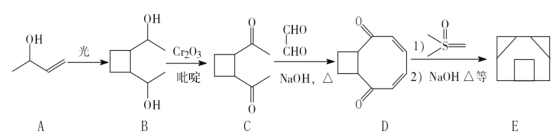
����Ŀ���л���A�ǾۺϷ�Ӧ�����������ϵĵ��壬�����Ϊ�ϳɵ����I����������J��ԭ�ϣ���غϳ�·�����£�
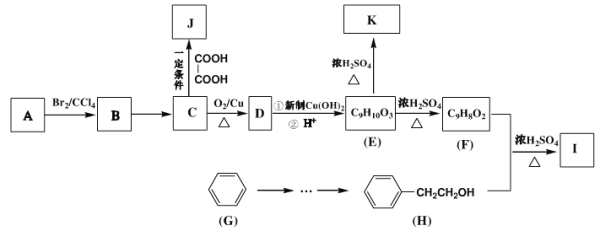
��֪��������ͼ����A������ʺɱ�Ϊ118���䱽���ϵ�һ�ȴ��ﹲ���֣��˴Ź���������ʾ�������Ϊ3:2:2:2:1��
����������Ϣ�ش��������⣺
(1)A�Ĺ���������Ϊ__________________��B��C�ķ�Ӧ����Ϊ_____________��E��F�ķ�Ӧ����Ϊ_____________��
(2)I�Ľṹ��ʽΪ____________________����K�����к���������Ԫ��״�ṹ���������ʽΪ________________��
(3)D������������ͭ����Һ��Ӧ�����ӷ���ʽΪ_______________________________��
(4)H��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������1 mol W���뷴Ӧ�������3 mol Br2����д�����з���������W�Ľṹ��ʽ___________________________________��
(5)J��һ�ָ߷��ӻ��������C����J�Ļ�ѧ����ʽΪ_____________________________________
���𰸡�̼̼˫�� ��������ˮ��Һ������ ��ȥ��Ӧ  C18H16O4
C18H16O4  +2Cu(OH)2+OH-
+2Cu(OH)2+OH-![]()
 +Cu2O+3H2O
+Cu2O+3H2O 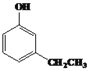 ��
�� n
n![]() +nHOOC-COOH
+nHOOC-COOH ![]()
 +(2n-1)H2O��
+(2n-1)H2O��
��������
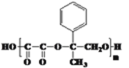
������ͼ����A������ʺɱ�Ϊ118������A����Է�������Ϊ118���仯ѧʽΪC9H10��������ͼ��֪A����Br2/CCl4����Һ�����ӳɷ�Ӧ��˵������̼̼˫��������䱽���ϵ�һ�ȴ��ﹲ���֣�˵��������ֻ��һ��ȡ���䣬���ݺ˴Ź���������ʾ�������Ϊ3��2��2��2��1����֪A�Ľṹ��ʽΪ![]() ����A��Br2/CCl4����Һ�����ӳɷ�Ӧ���ɵ�BΪ
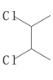
����A��Br2/CCl4����Һ�����ӳɷ�Ӧ���ɵ�BΪ ������NaOH��ˮ��Һ��ˮ�����ɵ�CΪ
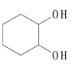
������NaOH��ˮ��Һ��ˮ�����ɵ�CΪ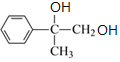 ��C���������ɵ�DΪ
��C���������ɵ�DΪ ��D��������Cu(OH)2��ϼ������ɵ�EΪ
��D��������Cu(OH)2��ϼ������ɵ�EΪ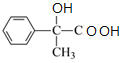 ��E��Ũ�������ʱ������ȥ��Ӧ����F��FΪ
��E��Ũ�������ʱ������ȥ��Ӧ����F��FΪ![]() ��F���뱽�Ҵ�����������Ӧ���ɵ���IΪ
��F���뱽�Ҵ�����������Ӧ���ɵ���IΪ ���ݴ˽��⡣
���ݴ˽��⡣
(1)A�Ľṹ��ʽΪ![]() �����еĹ�����Ϊ̼̼˫����
�����еĹ�����Ϊ̼̼˫���� ��NaOH��ˮ��Һ�����ˮ������C����B��C�ķ�Ӧ����ΪNaOHˮ��Һ�ͼ��ȣ�
��NaOH��ˮ��Һ�����ˮ������C����B��C�ķ�Ӧ����ΪNaOHˮ��Һ�ͼ��ȣ� ��Ũ�������ʱ�����ǻ�����ȥ��Ӧ����
��Ũ�������ʱ�����ǻ�����ȥ��Ӧ����![]() ����E��F�ķ�Ӧ����Ϊ��ȥ��Ӧ��
����E��F�ķ�Ӧ����Ϊ��ȥ��Ӧ��
(2)�ɷ�����֪I�Ľṹ��ʽΪ ����K�����к���������Ԫ��״�ṹ��˵��2���ӵ�
����K�����к���������Ԫ��״�ṹ��˵��2���ӵ� ͨ�����Ӽ�������Ӧ�����ɻ���K��2���ӵ�ˮ�����ԭ���غ㣬��֪K�ķ���ʽΪC18H16O4��
ͨ�����Ӽ�������Ӧ�����ɻ���K��2���ӵ�ˮ�����ԭ���غ㣬��֪K�ķ���ʽΪC18H16O4��
(3) ������Cu(OH)2��ϼ�������
������Cu(OH)2��ϼ������� �Ļ�ѧ����ʽΪ
�Ļ�ѧ����ʽΪ +2Cu(OH)2+OH-
+2Cu(OH)2+OH-![]()
 +Cu2O+3H2O��
+Cu2O+3H2O��
(4)![]() ��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������˵�����ӽṹ�к��з��ǻ���1molW���뷴Ӧ�������3molBr2����֪���ǻ����ڡ���λ���п�ȡ�����⣬�����������W�Ľṹ��ʽΪ
��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������˵�����ӽṹ�к��з��ǻ���1molW���뷴Ӧ�������3molBr2����֪���ǻ����ڡ���λ���п�ȡ�����⣬�����������W�Ľṹ��ʽΪ ��
��
(5)J��һ�ָ߷��ӻ�������� ���Ҷ��ᷢ�����۷�Ӧ����J�Ļ�ѧ����ʽn
���Ҷ��ᷢ�����۷�Ӧ����J�Ļ�ѧ����ʽn![]() +nHOOC-COOH
+nHOOC-COOH ![]()
 +(2n-1)H2O��
+(2n-1)H2O��