��Ŀ����
28.�������һ����Ҫ�ķǽ������ϣ��Ʊ��������Ҫ�������£��ٸ�������̼��ԭ���������Ƶôֹ�
�ڴֹ������HCl���巴Ӧ�Ƶ�SiHCl3��Si+3HCl![]() SiHCl3+H2
SiHCl3+H2
��SiHCl3�����H2��1000��1100�淴Ӧ�Ƶô���
��֪SiHCl3����H2Oǿ�ҷ�Ӧ���ڿ���������ȼ��
��ش��������⣺
(1)�ڢٲ��Ʊ��ֹ�Ļ�ѧ��Ӧ����ʽΪ_______________________________��
(2)�ֹ���HCl��Ӧ��ȫ�������õ���SiHCl3(�е�33.0��)�к�������SiCl4(�е�57.6��)��HCl(�е�-84.7��)���ᴿSiHCl3���õķ���Ϊ____________________��
(3)��SiHCl3�����H2��Ӧ�Ʊ������װ������(��Դ���г�װ����ȥ)��
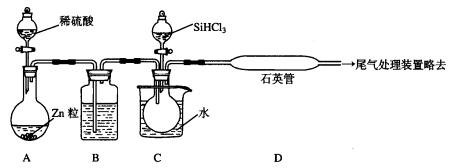
��װ��B�е��Լ���_________________________��
װ��C�е���ƿ��Ҫ���ȣ���Ŀ����_____________________________________��
�ڷ�Ӧһ��ʱ���װ��D�й۲쵽��������______________________________________��װ��D���ܲ�����ͨ�����ܵ�ԭ����____________________________________��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��Ϊ��֤�Ʊ�����ʵ��ijɹ��������Ĺؼ��Ǽ��ʵ��װ�õ������ԣ����ƺ÷�Ӧ�¶��Լ�______________________________________��
��Ϊ������Ʒ�����Ƿ��������ʣ���������ϡ�����ܽ⣬ȡ�ϲ���Һ�����ټ�����Լ�(��д��ĸ����)��_________________��
a.��ˮ b.��ˮ c.NaOH��Һ d.KSCN��Һ e.Na2SO3��Һ
��1��SiO2+2C![]() Si+2CO��
Si+2CO��
��2����������
��3����Ũ����
ʹ������ƿ�е�SiHCl3����
�������������
�ڷ�Ӧ�¶��£���ͨ����������
SiHCl3+H2![]() Si+3HCl
Si+3HCl
���ž�װ���еĿ���
��b d
��������1��������C��ԭSiO2����CO������CO2��
��2�����롢�ᴿ��ͬ�е��Һ����Բ��÷��������ķ�����
��3����Zn��ϡH2SO4�Ƶõ�H2���������H2O����SiHCl3����H2O���ҷ�Ӧ������Ҫ���и������װ��ѡ��Һ̬�����Ũ���ᡣCװ����ˮԡ����SiHCl3ʹ��ӷ������壬��H2����Dװ�÷�Ӧ����ΪSiHCl3��H2 Si��2HCl���¶Ⱥܸߣ�����ʹ����ͨ�����ܡ���ΪSiHCl3�����ڿ�������ȼ��ʵ���л�Ҫע����ͨһ��ʱ��H2�ž�װ���еĿ�����

��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣮
����д����̬��ԭ�ӵĺ�������Ų�ʽ______��
��NiO��FeO�ľ���ṹ���;����Ȼ�����ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO______�������������FeO��
��Ni��Fe��Co�Ƚ���������CO��Ӧ�γ�����Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������______��������ͣ�����λ����______��
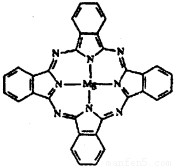
��2������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ��������ͼ���ü�ͷ��ʾ����λ����
��3��CO��N2��Ϊ�ȵ����壮CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ�������±����ݣ�˵��CO��N2���õ�ԭ����______��
| A-B | A=B | A��B | ||
| CO | ���� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵ | 441.2 273 | |||
| N2 | ���� | 154.8 | 418.3 | 941.7 |
| ���ܲ�ֵ | 263.6 523.3 | |||
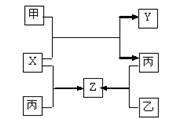
�ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��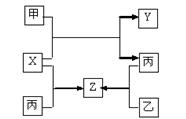
��֪���Ƕ����ڽ������ʣ��ҡ����Ƕ����ڷǽ������ʣ�X��Y��Z��ֻ��һ�������Ӿ��塣����˵������ȷ����
| A��X�Ǿ��м��Լ��ķǼ��Է��� | B��Z��ˮú������Ҫ�ɷ�֮һ |
| C����X�ķ�Ӧ�����ȷ�Ӧ | D���������������Ҫԭ�� |
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����