��Ŀ����
(9��) ��֪�й����ʵ�������ǿ��˳��Ϊ��Ũ���Fe3����H����Fe2�����Ƶ� 60 g ������100 mL Ũ�����ڼ��ȵ������·�Ӧ����Ӧ�����й���ʣ�࣬���ռ�����״��������22.4 L�����������Ϊ39.2 g���Իش��������⣺����֪��2Fe 3��+ Fe = 3Fe2����
��1���ڼ��ȵ������¿�ʼ��Ӧ�������й���ʣ�࣬���漰���Ļ�ѧ����ʽ�У�
_______________________________________________________________________��
��2����Ũ��������ʵ���Ũ�ȣ�
��3����Ӧ����ʣ��������������д���ļ�����̣�
��1���ڼ��ȵ������¿�ʼ��Ӧ�������й���ʣ�࣬���漰���Ļ�ѧ����ʽ�У�
_______________________________________________________________________��
��2����Ũ��������ʵ���Ũ�ȣ�
��3����Ӧ����ʣ��������������д���ļ�����̣�
��1��2Fe + 6H2SO4��Ũ��
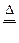 Fe2(SO4)3?+ 3SO2?+ 6H2O (2��)
Fe2(SO4)3?+ 3SO2?+ 6H2O (2��) Fe + H2SO4 = H2?+FeSO4 (2��)
��2��16 mol/L (3��)
��3�� 4 g (2��)
��

��ϰ��ϵ�д�
�����Ŀ

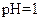 ����Һ�У�
����Һ�У�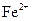 ��
��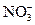 ��
��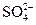 ��
��
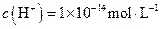 ����Һ�У�
����Һ�У�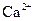 ��
�� ��
��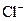 ��
��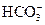
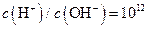 ����Һ�У�
����Һ�У�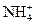 ��
��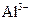 ��
�� ����Һ�У�
����Һ�У�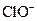 ��
��
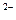 �����ʵ���Ũ��
�����ʵ���Ũ��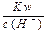 ="0.1" mol/L����Һ��Na+��K+��SiO32����NO3��
="0.1" mol/L����Һ��Na+��K+��SiO32����NO3��