��Ŀ����
 ���������ַ��������Ƶð�ɫ��Fe��OH��2������
���������ַ��������Ƶð�ɫ��Fe��OH��2������
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ�������______��
��2����ȥ����ˮ���ܽ��O2������______�ķ�����
��3�����ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������______��
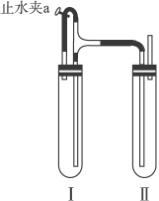
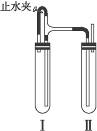
������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
��1�����Թܢ��������Լ���______��
��2�����Թܢ��������Լ���______��
��3��Ϊ���Ƶð�ɫFe��OH��2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����______��
��4���������ɵ�Fe��OH��2�����ܽϳ�ʱ�䱣�ְ�ɫ����������______��
�⣺����һ
��1������FeSO4��Һʱ�������ϡ�������м������Fe2+��ˮ�Ⲣ��ֹFe2+�������е�O2����ΪFe3+��
�ʴ�Ϊ��ϡH2SO4 ��м��
��2���������ˮ�ɳ�ȥ�����ܽ��O2���ʴ�Ϊ����У�
��3��Fe��OH��2�����ױ������е�����������ʵ��ʱ���ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������
FeSO4��ҺҺ���£��ټ���NaOH��Һ���ʴ�Ϊ���������ɵ� Fe��OH��2�����Ӵ�O2��
��������1���Թܢ����ṩ��ԭ����������������������Һ�������������м��Ӧ�����ɣ��ʴ�Ϊ��ϡH2SO4 ��м��
��2���Թܢ���ӦΪNaOH��Һ�����Թܢ������ɵ�FeSO4��Һ��Ӧ����Fe��OH��2�������ʴ�Ϊ��NaOH��Һ��
��3����ֹˮ�У�Fe��H2SO4��Ӧ����H2��������װ�ã���Ӧһ��ʱ���ر�ֹˮ�У�����Թ�����ѹ���ߣ���Ӧ���ɵ�Fe2+�ص��ܽ����Ҳ��Թ���NaOH��Ӧ���ɰ�ɫ����Fe��OH��2��������ر�ֹˮ�У�ʹ����Թ��е�����ѹ���Ҳ��Թ��У���NaOH�кͣ���ò���Fe��OH��2��Һ���ʴ�Ϊ�������Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ�У�
��4������װ���г���H2�������������룬���Գ����İ�ɫ��ά�ֽϳ�ʱ�䣬�ʴ�Ϊ���Թܢ��з�Ӧ���ɵ�H2�������Թܢ���Թܢ����������������룮
��������ʵ���������ַ������Ʊ�����������������һ��ȫ�Dz��ÿα��е�ʵ�飬��������������Һ�������е�Ҫ����Ҫע���ֹˮ������������Ʊ���������������Ҫ��ȥ�ܽ�����Һ�е��������Ʊ������������IJ���Ҫ�������ǶԿα�ʵ������죬��һ�ָĽ����Ʊ����������������������ķ�������֤���Ƶ�������������������������
������Fe��OH��2�����ױ������е���������������Fe��OH��2����Ҫ���ʣ���������ԭ�����ʻ����Ͻ����˸ı࣬��Ƴ���̽����ʵ���⣬���⿼������ݱȽ϶࣬����ˮ�ⷽ������⣬����������ԭ��������⣬����ʵ���е�ʵ�����⣬ͬʱ��������ʵ�����ƣ�����˵��һ�����ɶ�õĺ��⣮
��1������FeSO4��Һʱ�������ϡ�������м������Fe2+��ˮ�Ⲣ��ֹFe2+�������е�O2����ΪFe3+��
�ʴ�Ϊ��ϡH2SO4 ��м��
��2���������ˮ�ɳ�ȥ�����ܽ��O2���ʴ�Ϊ����У�
��3��Fe��OH��2�����ױ������е�����������ʵ��ʱ���ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������
FeSO4��ҺҺ���£��ټ���NaOH��Һ���ʴ�Ϊ���������ɵ� Fe��OH��2�����Ӵ�O2��
��������1���Թܢ����ṩ��ԭ����������������������Һ�������������м��Ӧ�����ɣ��ʴ�Ϊ��ϡH2SO4 ��м��
��2���Թܢ���ӦΪNaOH��Һ�����Թܢ������ɵ�FeSO4��Һ��Ӧ����Fe��OH��2�������ʴ�Ϊ��NaOH��Һ��
��3����ֹˮ�У�Fe��H2SO4��Ӧ����H2��������װ�ã���Ӧһ��ʱ���ر�ֹˮ�У�����Թ�����ѹ���ߣ���Ӧ���ɵ�Fe2+�ص��ܽ����Ҳ��Թ���NaOH��Ӧ���ɰ�ɫ����Fe��OH��2��������ر�ֹˮ�У�ʹ����Թ��е�����ѹ���Ҳ��Թ��У���NaOH�кͣ���ò���Fe��OH��2��Һ���ʴ�Ϊ�������Թܢ���ڴ��ų��������Ĵ��ȣ����ų���H2����ʱ���ټн�ֹˮ�У�
��4������װ���г���H2�������������룬���Գ����İ�ɫ��ά�ֽϳ�ʱ�䣬�ʴ�Ϊ���Թܢ��з�Ӧ���ɵ�H2�������Թܢ���Թܢ����������������룮
��������ʵ���������ַ������Ʊ�����������������һ��ȫ�Dz��ÿα��е�ʵ�飬��������������Һ�������е�Ҫ����Ҫע���ֹˮ������������Ʊ���������������Ҫ��ȥ�ܽ�����Һ�е��������Ʊ������������IJ���Ҫ�������ǶԿα�ʵ������죬��һ�ָĽ����Ʊ����������������������ķ�������֤���Ƶ�������������������������
������Fe��OH��2�����ױ������е���������������Fe��OH��2����Ҫ���ʣ���������ԭ�����ʻ����Ͻ����˸ı࣬��Ƴ���̽����ʵ���⣬���⿼������ݱȽ϶࣬����ˮ�ⷽ������⣬����������ԭ��������⣬����ʵ���е�ʵ�����⣬ͬʱ��������ʵ�����ƣ�����˵��һ�����ɶ�õĺ��⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 33�����������ַ��������Ƶð�ɫ��Fe��OH��2������
33�����������ַ��������Ƶð�ɫ��Fe��OH��2������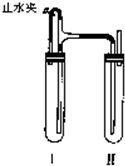 ��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2������
��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2������