��Ŀ����
��1��������ۻ������Ӽ�˷��� �������������������ۻ������Ӽ�˷��� ����������������������Ӽ�˷��� �������������־�����۵��ɸߵ��͵�˳���� ����2���������־��壺��CO2����NaCl����Na����Si����CS2�����ʯ�����ǵ��۵�ӵ͵��ߵ�˳��Ϊ ������ţ���
��3����H2����NH4��2SO4��SiC��CO2��HF�У��ɼ��Լ��γɵķǼ��Է����� ���ɷǼ��Լ��γɵķǼ��Է����� �����γɷ��Ӿ���������� ����������ľ���Ļ�ѧʽ�� ���������Ӿ������ ������ԭ�Ӿ������ ���������ʵ��۵��ɸߵ��͵�˳���� ��
��4��A��B��C��DΪ���־��壬�������£�
A����̬ʱ�ܵ��磬����������
B��������CS2��������ˮ
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ
D����̬��Һ̬ʱ�������磬�۵�Ϊ3 500��
���ƶ����ǵľ������ͣ�A�� ��B�� ��C�� ��D�� ��
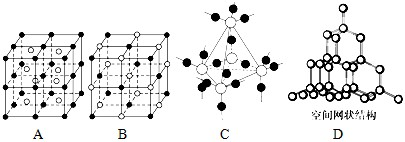
��5��ͼ��A��D����ѧ��ѧ�̿����ϳ����ļ��־���ṹģ�ͣ�����д��Ӧ���ʵ����ƣ�
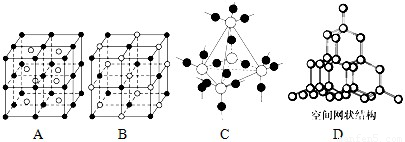
A�� ��B�� ��C�� ��D�� ��
���𰸡���������1������������Ӿ����ۻ�ʱ�ƻ����Ӽ�������������ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ������Ƿ��Ӿ��壬����ʱ���Ӽ�˷����Ӽ����������۵㣺ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
��2�������������ͷ�����ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
��3���������Ԫ�ؼ���֮��Ļ�ѧ��������
��4�����ݾ�����������ʷ�����
��5�����ݾ���ṹģ���ж����ʵ����ࣻ
����⣺��1������������Ӿ����ۻ�ʱ�ƻ����Ӽ�������������ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ������Ƿ��Ӿ��壬����ʱ���Ӽ�˷����Ӽ����������۵㣺ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬�����۵��С˳��Ϊ��SiO2��KClO3��I2���ʴ�Ϊ�����Ӽ� ���ۼ� ���Ӽ� SiO2��KClO3��I2��
��2�������������ͷ�����ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬Si��CS2����ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ���۵�Խ�ߣ������۵��С˳��Ϊ���٢ݢۢڢܢޣ��ʴ�Ϊ���٢ݢۢڢܢޣ�
��3���ɼ��Լ��γɵķǼ��Է�����CO2���ɷǼ��Լ��γɵķǼ��Է�����H2�����γɷ��Ӿ���������� H2��CO2��HF����������ľ���Ļ�ѧʽ�� HF���������Ӿ�����ǣ�NH4��2SO4������ԭ�Ӿ������SiC���������ʵ��۵��ɸߵ��͵�˳���� SiC����NH4��2SO4��HF��CO2��H2���ʴ�Ϊ��CO2 H2 H2��CO2��HF HF ��NH4��2SO4 SiC SiC����NH4��2SO4��HF��CO2��H2��
��4�����ݾ�����������ʷ�����
A����̬ʱ�ܵ��磬���������ᣬ���ڽ������壻
B��������CS2��������ˮ�����ڷ��Ӿ��壻
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ���������Ӿ��壻
D����̬��Һ̬ʱ�������磬�۵�Ϊ3 500�棬����ԭ�Ӿ��壻
�ʴ�Ϊ���������� ���Ӿ��� ���Ӿ��� ԭ�Ӿ��壻
��5���ɾ����ṹģ�Ϳ�֪A��B��C��D�ֱ�Ϊ�Ȼ�嵐��Ȼ��ơ��������衢���ʯ��
�ʴ�Ϊ���Ȼ�藍��Ȼ��ƣ��������裻���ʯ��
���������⿼���˾�����й����ʣ����ؿ���ѧ���������⡢��������������Ҳ��������ѧ������˼ά�����ͳ���˼ά������Ҳ�����ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ����ѧ����ѧϰЧ�ʣ�
��2�������������ͷ�����ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壻
��3���������Ԫ�ؼ���֮��Ļ�ѧ��������
��4�����ݾ�����������ʷ�����
��5�����ݾ���ṹģ���ж����ʵ����ࣻ
����⣺��1������������Ӿ����ۻ�ʱ�ƻ����Ӽ�������������ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ������Ƿ��Ӿ��壬����ʱ���Ӽ�˷����Ӽ����������۵㣺ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬�����۵��С˳��Ϊ��SiO2��KClO3��I2���ʴ�Ϊ�����Ӽ� ���ۼ� ���Ӽ� SiO2��KClO3��I2��
��2�������������ͷ�����ԭ�Ӿ��壾���Ӿ��壾���Ӿ��壬Si��CS2����ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ���۵�Խ�ߣ������۵��С˳��Ϊ���٢ݢۢڢܢޣ��ʴ�Ϊ���٢ݢۢڢܢޣ�
��3���ɼ��Լ��γɵķǼ��Է�����CO2���ɷǼ��Լ��γɵķǼ��Է�����H2�����γɷ��Ӿ���������� H2��CO2��HF����������ľ���Ļ�ѧʽ�� HF���������Ӿ�����ǣ�NH4��2SO4������ԭ�Ӿ������SiC���������ʵ��۵��ɸߵ��͵�˳���� SiC����NH4��2SO4��HF��CO2��H2���ʴ�Ϊ��CO2 H2 H2��CO2��HF HF ��NH4��2SO4 SiC SiC����NH4��2SO4��HF��CO2��H2��
��4�����ݾ�����������ʷ�����
A����̬ʱ�ܵ��磬���������ᣬ���ڽ������壻
B��������CS2��������ˮ�����ڷ��Ӿ��壻
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ���������Ӿ��壻
D����̬��Һ̬ʱ�������磬�۵�Ϊ3 500�棬����ԭ�Ӿ��壻
�ʴ�Ϊ���������� ���Ӿ��� ���Ӿ��� ԭ�Ӿ��壻
��5���ɾ����ṹģ�Ϳ�֪A��B��C��D�ֱ�Ϊ�Ȼ�嵐��Ȼ��ơ��������衢���ʯ��
�ʴ�Ϊ���Ȼ�藍��Ȼ��ƣ��������裻���ʯ��
���������⿼���˾�����й����ʣ����ؿ���ѧ���������⡢��������������Ҳ��������ѧ������˼ά�����ͳ���˼ά������Ҳ�����ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ����ѧ����ѧϰЧ�ʣ�

��ϰ��ϵ�д�
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
�����Ŀ