��Ŀ����
����ѡ����ʳ����ȷʹ��ҩ���DZ�֤���Ľ�������Ҫ���棬��������Щ���벻����ѧ����1��ά�����Dz������������������³´�л�������һ��С�����л��������������Ϥ��ά������A��B��C��D��E�ȣ�����ά����C�ֳ�
��2��ijͬѧһ���ʳ��Ϊ�������ӱ����������������Ǵ��Źǡ���ţ�⣮����Ϊ��λͬѧ��Ӧ�ó�һЩ
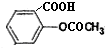
��3��������֪��һ�ֽ�����ʹҩ��˾ƥ�ֵĽṹʽΪ��
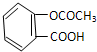
����д�����к��е�һ�ֹ����ŵ�����
��������1������ά����C�Ľṹ�����ʽ��н��
��2������Ӫ��������н��
��3�����ݰ�˾ƥ�ֵĽṹ���н��
��2������Ӫ��������н��
��3�����ݰ�˾ƥ�ֵĽṹ���н��
����⣺��1��ά����C�ܷ��λ�Ѫ�����ֳ�Ϊ����Ѫ�����ʹ��ɫʯ����Һ��죬˵��ά����C��Һ�������ԣ�
ά����C��������ϩ���Ľṹ���к�ǿ�Ļ�ԭ�ԣ�֬����ά���ذ���ά����A��D��E��K�ȣ�
�ʴ�Ϊ������Ѫ����ԭ��ά����A��D��E��
��2���������Ҫ������ۡ������ʵ�Ӫ�������⣬��������һ��������ά�أ��ʻ�Ӧ�ó�һЩˮ���߲���ʳ�
�ʴ�Ϊ��ˮ���߲ˣ�
��3���ɰ�˾ƥ�ֵĽṹ��֪����˾ƥ�ֺ����Ȼ����������Ȼ�����NaHCO3��Һ��Ӧ���������ƶ�����̼��ˮ������ʽΪ��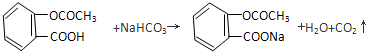 ��
��
�ʴ�Ϊ���Ȼ��� ��
��
ά����C��������ϩ���Ľṹ���к�ǿ�Ļ�ԭ�ԣ�֬����ά���ذ���ά����A��D��E��K�ȣ�
�ʴ�Ϊ������Ѫ����ԭ��ά����A��D��E��
��2���������Ҫ������ۡ������ʵ�Ӫ�������⣬��������һ��������ά�أ��ʻ�Ӧ�ó�һЩˮ���߲���ʳ�
�ʴ�Ϊ��ˮ���߲ˣ�
��3���ɰ�˾ƥ�ֵĽṹ��֪����˾ƥ�ֺ����Ȼ����������Ȼ�����NaHCO3��Һ��Ӧ���������ƶ�����̼��ˮ������ʽΪ��
 ��
���ʴ�Ϊ���Ȼ���
 ��
�����������⿼��ά����C�Ľṹ�����ʡ�ά���صķ��ࡢ��˾ƥ�ֵ������Լ�����ʽ����д�ȣ��ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ���dz��õ�
���dz��õ�