��Ŀ����
����������벻����ѧ��ӵ�л�ѧ֪ʶ����ʹ��������ø������š�
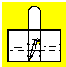
��1��������������������ͷ�չ����Ҫ���ʻ�����
��һ������£��Ͻ��������ijɷֽ���Ӳ��_________�����С������
��__________��ʴ����ɸ�����ʴ����Ҫԭ��ͨ�����ں�ˮ����ʻ���ִ�������װ��һ��������_________���п����ͭ����������ֹ�����ĸ�ʴ�����г��ĸ�Ȧͨ������_________���Ϳ���ᡱ��Cr��������ʴ��
��2��Ϊ�˼���úȼ�ղ�����SO2�Դ�����ɵ���Ⱦ������ú�м�������ʯ��ʯ��ʯ��ʯ��úȼ��ʱ������SO2�������е�O2��Ӧ������CaSO4��CO2��д���÷�Ӧ�Ļ�ѧ����ʽ___________________��
��3������ѡ����ʳ����ȷʹ��ҩ����������õ�����ϰ�ߣ��DZ�֤���Ľ�������Ҫ���档
����������ʳ����࣬������ѪҺƫ�ᣬ�����γɡ�����֢������������ȱ�ơ�ѪҺճ������ȣ�������Ҫ������������ʳ������ʳ�������������ʳ�����_______������ĸ����
a ���� b ���ܲ� c ���� d ����
�ڿ���ҩ����������к�θ���������ᡣij����ҩ����Ҫ�ɷ���̼��ƣ�д��̼��������ᷴӦ�����ӷ���ʽ��_______________�����˿���ҩÿƬ��̼���0.50 g��ȡ��ҩһƬ����������������ᷴӦ�����������ڱ�״���µ����Ϊ_________mL��
��1��������������������ͷ�չ����Ҫ���ʻ�����
��һ������£��Ͻ��������ijɷֽ���Ӳ��_________�����С������
��__________��ʴ����ɸ�����ʴ����Ҫԭ��ͨ�����ں�ˮ����ʻ���ִ�������װ��һ��������_________���п����ͭ����������ֹ�����ĸ�ʴ�����г��ĸ�Ȧͨ������_________���Ϳ���ᡱ��Cr��������ʴ��
��2��Ϊ�˼���úȼ�ղ�����SO2�Դ�����ɵ���Ⱦ������ú�м�������ʯ��ʯ��ʯ��ʯ��úȼ��ʱ������SO2�������е�O2��Ӧ������CaSO4��CO2��д���÷�Ӧ�Ļ�ѧ����ʽ___________________��
��3������ѡ����ʳ����ȷʹ��ҩ����������õ�����ϰ�ߣ��DZ�֤���Ľ�������Ҫ���档
����������ʳ����࣬������ѪҺƫ�ᣬ�����γɡ�����֢������������ȱ�ơ�ѪҺճ������ȣ�������Ҫ������������ʳ������ʳ�������������ʳ�����_______������ĸ����
a ���� b ���ܲ� c ���� d ����
�ڿ���ҩ����������к�θ���������ᡣij����ҩ����Ҫ�ɷ���̼��ƣ�д��̼��������ᷴӦ�����ӷ���ʽ��_______________�����˿���ҩÿƬ��̼���0.50 g��ȡ��ҩһƬ����������������ᷴӦ�����������ڱ�״���µ����Ϊ_________mL��
��1���ٴڵ绯ѧ������������п����Cr
��2��2CaCO3 + O2 + 2SO2 2CaSO4 + 2CO2
2CaSO4 + 2CO2
��3����cd����CaCO3+2H+= Ca2+ + CO2��+H2O��112
��2��2CaCO3 + O2 + 2SO2
 2CaSO4 + 2CO2
2CaSO4 + 2CO2 ��3����cd����CaCO3+2H+= Ca2+ + CO2��+H2O��112

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����������벻����ѧ��ӵ�л�ѧ֪ʶ����ʹ��������ø������ţ�
����������벻����ѧ��ӵ�л�ѧ֪ʶ����ʹ��������ø������ţ�