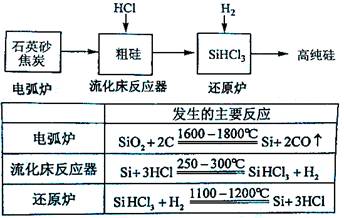
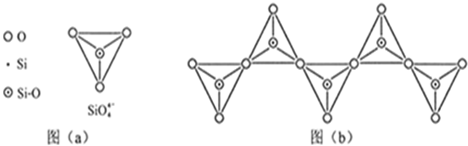
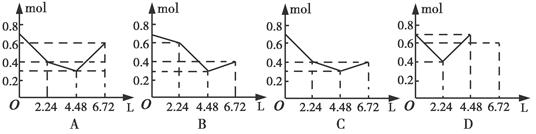
��Ŀ����
����˵����ȷ����
| A�������ķ��������ݻ�����и�������ʵIJ�����еģ�Ԫ�ط����ǡ���������ǡ�ԭ�����չ����ǵȾ��Ƿ������ʵij��������� |
| B�������١���ķ����¬ɪ���������������ӵ��ȿ�ѧ����ԭ�ӽṹ����ʶ���������ش��ף�������Ҫ���õ��Ƕ����о���ʵ���о��ķ����� |
| C��ú��ʯ�͵��ۺ�������ú��������ú��Һ����ʯ�͵ķ����������仯��ʯ�͵��ѻ����ѽ⡢ú�ĸ����ǻ�ѧ�仯 |
| D��ijЩ���������γɵķ���ɸ����������״��Ѩ��ͨ���������ڷ��롢�ᴿ�����Һ���������������������ӽ���������������������� |
D
���������A��Ԫ�ط����ǡ���������ǡ�ԭ�����չ����ǵȾ��Dzⶨ������ɺͽṹ�ij������������� B�������١���ķ����¬ɪ���������������ӵ��ȿ�ѧ����ԭ�ӽṹ����ʶ���������ش��ף�������Ҫ���õ��Ǽ�˵��ģ�͵ķ���������C��ú��ʯ�͵��ۺ�������ʯ�͵ķ����������仯��ʯ�͵��ѻ����ѽ⡢ú�ĸ���ú��������ú��Һ�����ǻ�ѧ�仯������D��ijЩ���������γɵķ���ɸ����������״��Ѩ��ͨ���������ڷ��롢�ᴿ�����Һ���������������������ӽ���������������������ȣ���ȷ��

��ϰ��ϵ�д�
�����Ŀ