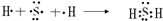��Ŀ����
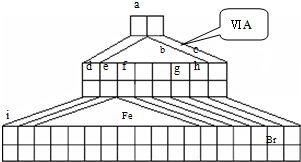
I����ͼ����Emil Zmaczynski��ƵĽ�����ʽԪ�����ڱ���һ���֣�ͼ�ϱ��еڢ�A��ͼ���Ԫ�ص�λ�ã���ش��������⣺
��1���Դ����ڱ�����������������Ԫ�������ڱ��е�λ�ã�һֱ���ڷ������������Ű������ڵڢ�A�壬�����ǣ�����������ӡ�����ȱһ������д��NaH�ĵ���ʽ______��
��2��Fe�������______
��3���õ���ʽ��ʾԪ��a��g�γɻ�������γɹ���______��
��4��д��bԪ�ص��⻯����bԪ�ص�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ______
��5��d������������Ӧ��ˮ������f�ĵ��ʷ�Ӧ�����ӷ���ʽ______��
��6��aԪ����̼Ԫ���γɷ��ӵĿռ�ṹ�����ǣ�����ţ�______��

��7�������ʵ��Ƚ�e f �Ľ�����ǿ����Ҫ���в����������ۣ�______��
��8��d��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ���ɺ��ɫ������ij���壬����������������ʵ���֮��Ϊ2��1����Ӧ�����ӷ���ʽΪ______��

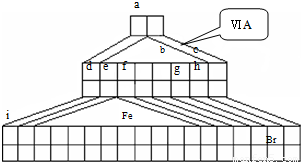
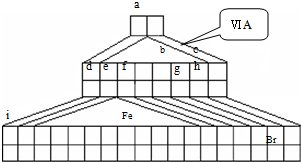
��1���Դ����ڱ�����������������Ԫ�������ڱ��е�λ�ã�һֱ���ڷ������������Ű������ڵڢ�A�壬�����ǣ�����������ӡ�����ȱһ������д��NaH�ĵ���ʽ______��
��2��Fe�������______
��3���õ���ʽ��ʾԪ��a��g�γɻ�������γɹ���______��
��4��д��bԪ�ص��⻯����bԪ�ص�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ______
��5��d������������Ӧ��ˮ������f�ĵ��ʷ�Ӧ�����ӷ���ʽ______��
��6��aԪ����̼Ԫ���γɷ��ӵĿռ�ṹ�����ǣ�����ţ�______��

��7�������ʵ��Ƚ�e f �Ľ�����ǿ����Ҫ���в����������ۣ�______��
��8��d��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ���ɺ��ɫ������ij���壬����������������ʵ���֮��Ϊ2��1����Ӧ�����ӷ���ʽΪ______��
��1����H���ڵڢ�A�壬��HԪ�ص���ͻ��ϼ�Ϊ-1�ۣ���NaHΪ���ӻ��������ʽΪNa+[��H]-���ʴ�Ϊ��Na+[��H]-��
��2����Ԫ���ڵ������ڣ������ڱ��еĵ�8�У����������ڵ�VIII�壬�ʴ�Ϊ���������ڵ�VIII�壻
��3������������ԭ�Ӻ���ԭ�Ӽ�ͨ�����õ��Ӷ��γɵĹ��ۻ�������γɹ��̱�ʾΪ
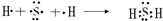
���ʴ�Ϊ��
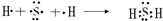
��
��4�������ڱ���֪��bΪNԪ�أ�bԪ�ص��⻯�ﰱ����������������ˮ�������������һ����Ϊ����泥���NH3+HNO3�TNH4NO3���ʴ�Ϊ��NH3+HNO3�TNH4NO3��
��5��d������������������ƺ�f�ĵ�������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��6��̼ԭ�Ӻ���ԭ��֮���γɵĻ������������������ṹ������ƽ�������νṹ���ʴ�Ϊ��cd��
��7���Ƚ�þ��������������ǿ���ķ�����ȡ��С��ȵ�þ�������������뵽��ͬŨ�ȵ������У��۲���������Ŀ��������Ȳ��������Ľ���������ǿ����֮�����������ʴ�Ϊ��ȡ��С��ȵ�þ�������������뵽��ͬŨ�ȵ������У��۲���������Ŀ��������Ȳ��������Ľ���������ǿ����֮����������
��8��dΪ�ƣ�d��ij������ʵ���ɫ����û�����Ϊ�������ƣ����������ԣ���������������ʵ���֮��Ϊ2��1�������ķ�ӦΪ3Na2O2+4H2O+2Fe2+�T2Fe��OH��3+O2��+6Na++2OH-��
�ʴ�Ϊ��3Na2O2+4H2O+2Fe2+�T2Fe��OH��3+O2��+6Na++2OH-��
��2����Ԫ���ڵ������ڣ������ڱ��еĵ�8�У����������ڵ�VIII�壬�ʴ�Ϊ���������ڵ�VIII�壻
��3������������ԭ�Ӻ���ԭ�Ӽ�ͨ�����õ��Ӷ��γɵĹ��ۻ�������γɹ��̱�ʾΪ
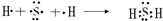
���ʴ�Ϊ��
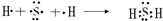
��
��4�������ڱ���֪��bΪNԪ�أ�bԪ�ص��⻯�ﰱ����������������ˮ�������������һ����Ϊ����泥���NH3+HNO3�TNH4NO3���ʴ�Ϊ��NH3+HNO3�TNH4NO3��
��5��d������������������ƺ�f�ĵ�������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��6��̼ԭ�Ӻ���ԭ��֮���γɵĻ������������������ṹ������ƽ�������νṹ���ʴ�Ϊ��cd��
��7���Ƚ�þ��������������ǿ���ķ�����ȡ��С��ȵ�þ�������������뵽��ͬŨ�ȵ������У��۲���������Ŀ��������Ȳ��������Ľ���������ǿ����֮�����������ʴ�Ϊ��ȡ��С��ȵ�þ�������������뵽��ͬŨ�ȵ������У��۲���������Ŀ��������Ȳ��������Ľ���������ǿ����֮����������
��8��dΪ�ƣ�d��ij������ʵ���ɫ����û�����Ϊ�������ƣ����������ԣ���������������ʵ���֮��Ϊ2��1�������ķ�ӦΪ3Na2O2+4H2O+2Fe2+�T2Fe��OH��3+O2��+6Na++2OH-��
�ʴ�Ϊ��3Na2O2+4H2O+2Fe2+�T2Fe��OH��3+O2��+6Na++2OH-��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ