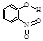��Ŀ����
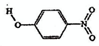
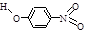
��2�������γɷ��Ӽ�����ͷ�����������������ʵ�Ӱ�����������죮�����±����ݣ��γɷ��Ӽ������������
| ���� | ���� | �ṹ��ʽ | ˮ���ܽ��/g ��25�棩 |
�۵�/�� | �е�/�� |
| A | ��-�������� |  |
0.2 | 45 | 100 |
| B | ��-�������� |  |
1.7 | 114 | 295 |
�ǽ�����Խǿ���γ��⻯��Ļ�ѧ������Խ��ͬһ���ڣ��������Ԫ�صķǽ�������ǿ���ݴ��ж�Ԫ�أ���д�⻯�ﻯѧʽ��
��2���γɷ��Ӽ�����۷е���γɷ�����������۷е���ߣ���ˮ�е��ܽ�ȸ���
��3��ͬһ���ڣ��������Ԫ�ص縺������ԭ�Ӱ뾶ԽС������Խ�̣����ۼ�Խ�ȶ�������Խ��
�ǽ�����Խǿ���γ��⻯��Ļ�ѧ������Խ��ͬһ���ڣ��������Ԫ�صķǽ�������ǿ���ڶ����ڷǽ�������ǿ��Ԫ����F��HF�л�ѧѧ���������
�ʴ�Ϊ��Na��3s1��HF��
��2���γɷ��Ӽ�����۷е���γɷ�����������۷е���ߣ���ˮ�е��ܽ�ȸ����ɱ������ݿ�֪����-�������ӱ���-����������ˮ���ܽ�ȴ��۷е�ߣ����γɷ��Ӽ�����������Ƕ�-�������ӣ��ʴ�Ϊ��B��
��3��ͬһ���ڣ��������Ԫ�ص縺���������Ե縺�ԵĴ�С��C��O��ԭ�Ӱ뾶Br��I������H-Br��H-I������Խ�̣����ۼ�Խ�ȶ�������Խ�ʼ��ܵĴ�С��HBr��HI��
�ʴ�Ϊ����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�A�������ʽṹ�����ʡ�
��1��ǰ������Ԫ���е�һ��������С���� _______ (��Ԫ�ط���)�����̬ԭ�ӵĵ����Ų�ʽΪ _______ ���ڶ����ڷǽ���Ԫ���γɵ��⻯���л�ѧ������������ _______
(�����ʽ)����������CCl4�е��ܽ�ȱ���ˮ�е��ܽ�� _______ (���С��)��
 |
��2�������γɷ��Ӽ�����ͷ�����������������ʵ�Ӱ�����������졣�����±����ݣ��γɷ��Ӽ������������ _______ (��������ĸ����)��
��3�������ܵĴ�С��MgO _______ NaCl�����ܵĴ�С��HBr _______ HI��(�>������������<��)
��4���������ʵ��۵�ߵ�˳����ȷ���� _______
A�����ʯ>�����>��������>̼����
B��CI4 > CBr4 > CCl4 > CH4
C��SiF4 > NaF > NaCl > NaBr
B��ʵ�黯ѧ��
��ˮ����þ(MgSO4��7H2O)��ӡȾ����ֽ��ҽҩ�ȹ�ҵ�϶��й㷺��Ӧ�ã����û�����������ɰ�ķ�����һ��þ�����ȡ��ˮ����þ����þ�����Ҫ�ɷ���MgCO3����������������(MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1 ����������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Mg(OH)2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
��2 �����ε��ܽ��(��λΪg��100gˮ)
| �¶� / �� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
��þ����ȡ��ˮ����þ�Ĺ����������£�

������������ͼ���ο�����pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1������I����Һ�м�����þ�࣬������Һ��pH��5��6���ټ���NaClO��Һ������У�����Һ�е�Mn2+������MnO2����Ӧ�����ӷ�Ӧ����ʽΪ _______ ��������е���ҪĿ����_ _______ ��
��2������B�г�MnO2��SiO2����� _______ (�ѧʽ)�����ʡ�
��3��������ˢ�����Һ���Ƿ���Fe3����ʵ�鷽���� _______ ��
��4������C�Ļ�ѧʽ�� _______ ������II����ȹ��˵������� _______
��1��ǰ������Ԫ���е�һ��������С����___________ (��Ԫ�ط���)�����̬ԭ�ӵĵ����Ų�ʽΪ______________________���ڶ����ڷǽ���Ԫ���γɵ��⻯���л�ѧ������������__________(�����ʽ)��
��2�������γɷ��Ӽ�����ͷ�����������������ʵ�Ӱ�����������졣�����±����ݣ��γɷ��Ӽ������������___________ (��������ĸ����)��
|
���� |
���� |
�ṹ��ʽ |
ˮ���ܽ��/g ��25�棩 |
�۵�/�� |
�е�/�� |
|
A |
�ڡ��������� |
|
0.2 |
45 |
100 |
|
B |
�ԡ��������� |
|
1.7 |
114 |
295 |
��3���縺�ԵĴ�С��C___________O�����ܵĴ�С��HBr___________HI��(�����������������)