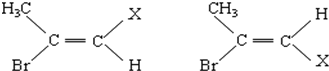��Ŀ����
5����Է�������������100���л���A������������Ʒ�Ӧ������ɫ���壬������̼���Ʒ�Ӧ������ɫ���壬������ʹ������Ȼ�̼��Һ��ɫ�� A��ȫȼ��ֻ����CO2��H2O���������京��Ԫ�ص���������Ϊ37.21%������A��Է�������Ϊ86�� �������������ȡ������
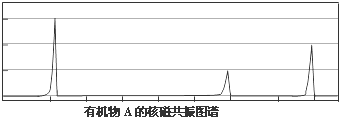
���˴Ź����ⷢ��A��ͼ�����£�
����д��A�Ľṹ��ʽ��CH2=C��CH3��COOH��
����д��A��״���Ӧ�����л���B�Ļ�ѧ��Ӧ����ʽ��CH2=C��CH3��COOH+CH3OH$?_{һ������}^{��Ũ����}$CH2=C��CH3��COOCH3+H2O��
��B��һ�������¿��Է�Ӧ���ɸ߷��ӻ�����-�л�������д����Ӧ�ķ���ʽ��nCH2=C��CH3��COOCH3$\stackrel{һ������}{��}$
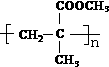 ��
����B��ͬ���칹���ж��֣���д��һ�ֺ�Bͬ����ͬ���칹��Ľṹ��ʽCH2=CHCOOCH2CH3��
���� �ٸ��ݺ���Ԫ�ص���������Ϊ37.21%����Է�������������100��ȷ����������ԭ�Ӹ���������ȷ���л������Է���������
���л����������Na��Ӧ������ɫ���壬˵������-OH��-COOH��������̼���Ʒ�Ӧ������ɫ���壬˵������-COOH��������ʹ������Ȼ�̼��Һ��ɫ��˵�����в����ͼ� ��-C��C-��
��-C��C-��
��AΪ���ᣬA��״���Ӧ��������ˮ���״��γɷ�Ӧ�Ļ�ѧ����ʽ��
��B��2-����ϩ������Ӿ۷�Ӧ�����л�������Ȼ��д����Ӧ���ɾ�2-����ϩ������ķ���ʽ��
�ݸ���B�ķ�����ɼ����Ҫ��д�������������л���Ľṹ��ʽ��
��� �⣺���л�������Ԫ�ص���������Ϊ37.21%����Է�������������100�����Է�������ԭ����ĿN��O����$\frac{100��37.21%}{16}$��2.3���л�������̼���Ʒ�Ӧ������ɫ���壬˵��������к���-COOH������A�к���2����ԭ�ӣ�Mr��X��=$\frac{16��2}{37.21%}$=86���ʴ�Ϊ��86��
���л���X�����Ȼ�����ȫȼ��ֻ����CO2��H2O��˵���л���X��C��H��O����Ԫ����ɣ�
�����������ΪCnHm����������Է�������Ϊ86-45=41������12n+m=41��
��n=1����m=29�������ϣ�����n=2����m=17�������ϣ�����n=3����m=5�����ϣ���
�������������ΪC3H5��A�Ľṹ��ʽΪ��C3H5-COOH��
�ʴ�Ϊ��CH2=C��CH3��COOH��
��A�Ľṹ��ʽΪCH2=C��CH3��COOH����״���Ӧ�Ļ�ѧ����ʽΪ��CH2=C��CH3��COOH+CH3OH $?_{һ������}^{��Ũ����}$ CH2=C��CH3��COOCH3+H2O��
�ʴ�Ϊ��CH2=C��CH3��COOH+CH3OH $?_{һ������}^{��Ũ����}$ CH2=C��CH3��COOCH3+H2O��
��2-����ϩ�������Ӧ�����л�������2-����ϩ������ķ���ʽΪ��nCH2=C��CH3��COOCH3$\stackrel{һ������}{��}$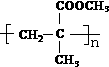 ��
��
�ʴ�Ϊ��nCH2=C��CH3��COOCH3$\stackrel{һ������}{��}$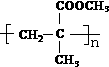 ��
��
��BΪ2-����ϩ���������B��Ϊͬ���ͬ���칹����л�����Է��ӽṹ�к���������̼̼˫��������Bͬ����ͬ���칹��Ľṹ��ʽ��CH3CH=CHCOOCH3��CH2=CHCH2COOCH3��CH2=CHCOOCH2CH3���ʴ�Ϊ��CH2=CHCOOCH2CH3��
���� ���⿼�����л������ʽ���ṹ��ʽ��ͬ���칹�����д����Ŀ�Ѷ��еȣ�ע�����ճ����л������ʽ���ṹ��ʽ�ķ�������ȷͬ���칹�����д������

| A�� | ��⡢��ơ��绯ѧ��ʴ����Ҫͨ��ſɽ��� | |
| B�� | Al��Fe��Cu���߶�Ӧ���������Ϊ���������� | |
| C�� | ŨH2SO4��������ˮ�ֱ����pH��ֽ�Ͼ�������ȱ�����ɫ������ | |
| D�� | �������������к����θ�� |
| A�� | ���ױ�������ͬ���칹�� | |
| B�� | ���ױ��ͼױ���ͬϵ�� | |
| C�� | ���ױ������У�����̼ԭ�Ӷ���ͬһ��ƽ���� | |
| D�� | 1mol���ױ���ȫȼ�պ����ɵĶ�����̼��ˮ�����ʵ������ |
| A�� | ����ϩ���ϵ��ϻ������ڷ����˼ӳɷ�Ӧ | |
| B�� | �ع��͵���Ҫ�ɷ�����֬������������͡�ú����ͬ | |
| C�� | �ϳ���ά��������ά�����ά�������л��߷��Ӳ��� | |
| D�� | ������ʳ��ƾ����˵��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� |