��Ŀ����
�±�ΪԪ�����ڱ������ڵ�һ���֡��ش��������⣺
| | | | | |
| A | | D | | |
| | | E | G | M |
��2����ָ��G�ķǽ����Ա�E�ķǽ�����ǿ����ʵ��д3�� ��
 ��3��E��GԪ�ص�ԭ�Ӿ����γ���Mԭ�ӵ��Ӳ�ṹ��ͬ�ļ����ӣ���E���ӵİ뾶��������� ��
��3��E��GԪ�ص�ԭ�Ӿ����γ���Mԭ�ӵ��Ӳ�ṹ��ͬ�ļ����ӣ���E���ӵİ뾶��������� ����4��A����Ԫ���γɷ��ӵĿռ�ṹ�����ǣ�ѡ����ţ� ��
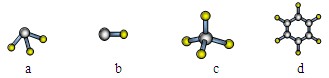
������8�֣�
��1��3p��1�֣������������������ͨ�ԣ��������������л�ԭ�ԣ��������ɣ���2�֣���д���o�֣�
��2��G���⻯����ȶ���G����ߺ����� ���Ը�ǿ��G�ĵ����ܰ�E�ĵ����û��������������ɣ���2�֣���д1����1�֣�
���Ը�ǿ��G�ĵ����ܰ�E�ĵ����û��������������ɣ���2�֣���д1����1�֣�
��3��G���ӵĺ˵�������������ӵ�������ǿ��1�֣�
��4��c d ��2�֣���ѡ��1�֣���ѡ�����֣�
����

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д���16�֣� A��B��C��D��E��F��Ԫ�����ڱ������ֶ�����Ԫ�أ�����ݱ�����Ϣ�ش���������
| Ԫ�� | A | B | C | D | E | F |
|
���ʻ�ṹ ��Ϣ | �γɻ�������������Ԫ�� | �������������۾���ֵ��3�� | ������������������3�� | ����������������3�� | �ؿ��к�����ߵĽ���Ԫ�� | ����������Ӧˮ��������ǿ�ĺ����� |
��1��Ԫ��B �����ӽṹʾ��ͼ Ԫ��F�����������Ļ�ѧʽΪ ��
��2��Ԫ��A��D�ֱ���Ԫ��C �γ�ԭ�Ӹ�����Ϊ1��2�Ļ������1��1 �Ļ������ң�������� ���ӣ�����ԡ��Ǽ��ԡ������������ҵĵ���ʽ �������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3��Ԫ��B��D��ɻ���������侧������Ϊ �������������Һ�� ������ԡ������ԡ������ԡ�����
��4��Ԫ��A����Ԫ����ɵ���Ļ����ﶡ��һ������ȼ�ϣ�ȼ����ֵ�ߡ�
��ʵ���ã��ڳ��³�ѹ�£�1g�����ﶡ��ȫȼ������Һ̬ˮ���ų�55.65 kJ ���������ʾ�����ﶡ��ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��ijʵ��С�����ݶ�ȼ�յķ�Ӧԭ�����������ͼ��ʾ�ĵ��װ�á�
a���õ�������ĵ缫��ӦΪ ��
b������һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�����ӷ���ʽΪ ��
��5��GҲ�Ƕ�����Ԫ�أ��������Ӳ�����������������ȣ�����E����Ԫ�����ڱ��жԽ���λ�ã����ǵĵ��ʼ��仯������������ƣ�д��G����������NaOH��Һ��Ӧ�����ӷ���ʽ�� ����E���Ȼ����۷е�ϵ͡�����������E���Ȼ����� ���������ӡ����ۡ����������һ����ʵ��֤�����ѡ�� ��
��16�֣� A��B��C��D��E��F��Ԫ�����ڱ������ֶ�����Ԫ�أ�����ݱ�����Ϣ�ش���������
|
Ԫ�� |
A |
B |
C |
D |
E |
F |
|
���ʻ�ṹ ��Ϣ |
�γɻ�������������Ԫ�� |
�������������۾���ֵ��3�� |
������������������3�� |
����������������3�� |
�ؿ��к�����ߵĽ���Ԫ�� |
����������Ӧˮ��������ǿ�ĺ����� |
��1��Ԫ��B �����ӽṹʾ��ͼ Ԫ��F�����������Ļ�ѧʽΪ ��
��2��Ԫ��A��D�ֱ���Ԫ��C �γ�ԭ�Ӹ�����Ϊ1��2�Ļ������1��1 �Ļ������ң�������� ���ӣ�����ԡ��Ǽ��ԡ������������ҵĵ���ʽ �������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3��Ԫ��B��D��ɻ���������侧������Ϊ �������������Һ�� ������ԡ������ԡ������ԡ�����
��4��Ԫ��A����Ԫ����ɵ���Ļ����ﶡ��һ������ȼ�ϣ�ȼ����ֵ�ߡ�
��ʵ���ã��ڳ��³�ѹ�£�1g�����ﶡ��ȫȼ������Һ̬ˮ���ų�55.65 kJ ���������ʾ�����ﶡ��ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��ijʵ��С�����ݶ�ȼ�յķ�Ӧԭ�����������ͼ��ʾ�ĵ��װ�á�

a���õ�������ĵ缫��ӦΪ ��
b������һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�����ӷ���ʽΪ ��
��5��GҲ�Ƕ�����Ԫ�أ��������Ӳ�����������������ȣ�����E����Ԫ�����ڱ��жԽ���λ�ã����ǵĵ��ʼ��仯������������ƣ�д��G����������NaOH��Һ��Ӧ�����ӷ���ʽ�� ����E���Ȼ����۷е�ϵ͡�����������E���Ȼ����� ���������ӡ����ۡ����������һ����ʵ��֤�����ѡ�� ��
��17�֣������±����ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���û�ѧ����ش��������⡣
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
R |
Ԫ��������������������������ԭ����������� |
|
T |
�����������Ǵ�����������2�� |
|
X |
Ԫ���������+7�� |
|
Y |
�������ڽ���Ԫ����ԭ�Ӱ뾶��С |
|
Z |
�����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
��1��д��Ԫ��T��ԭ�ӽṹʾ��ͼ ��Ԫ��Z���ʵĵ���ʽ ��д��X�����ڱ��е�λ�� ; Ԫ��T�����������Ľṹʽ .
��2��̽Ѱ���ʵ����ʲ�����ѧϰ����Ҫ����֮һ������T��X��Y��Z����Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������
�� ���ѧʽ���������� ��
��3��д��R��T��X������Ԫ���е�ij����Ԫ���γɵĻ������У�ÿ��ԭ�Ӷ����������Ϊ8�����ȶ��ṹ��һ�����ʵĻ�ѧʽ
��4���ɱ���Ԫ���γɵij�������A��B��C��D��E�ɷ������·�Ӧ��

A��Һ��B��Һ���ʼ��ԣ���Ӧ�����ӷ���ʽΪ ��
D�к��л�ѧ���������� ������ ������ӡ����ۡ��������
X��Y��Z��Q��W����Ԫ�ص�ԭ���������ε�������֪��
��W��ԭ������Ϊ29������ľ�Ϊ����������Ԫ�أ�
��Xԭ�ӵļ۵����Ų�Ϊnsnnpn��
��YԪ���ǿ����к�������Ԫ�أ�
��ZԪ�صĵ縺�Դ���YԪ�أ�Zԭ�Ӻ���ijɶԵ�������δ�ɶԵ�����֮��Ϊ3:1��
��QԪ�صĵ��������ݼ��±�����λ��kJ/mol����
|
I1 |
I2 |
I3 |
I4 |
I5[] |
...... |
|
578 |
1817 |
2745 |
11575 |
14380 |
...... |



 ��1��
��1��

 WԪ��λ�����ڱ���
����Z2+ �ĺ�������Ų�ʽ��
��
WԪ��λ�����ڱ���
����Z2+ �ĺ�������Ų�ʽ��
��
��2��X��Y��Z����Ԫ�صĵ�һ��������ֵ��С�����˳��Ϊ ����Ԫ�ط������𣩡�
��3��QԪ�������ڱ��е�λ��Ϊ ���������������������������ȶ���̬Ϊ ��
��4��������,Y���ʵĻ�ѧ���ʺ��ȶ���ԭ���� ��
��5��X��һ���⻯����Է�������Ϊ26�����з����еĦҼ���м��ļ���֮��Ϊ ��
