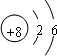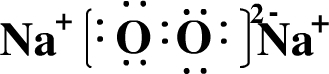��Ŀ����
��A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
Ԫ�ر�� Ԫ��������Ϣ
A A�ĵ������ܶ���С������
B B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������
C C��ԭ�����������������ڲ������������
D D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С
E B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ�
F FԪ���������������۵Ĵ�����Ϊ4
��1��д�����־���A��B��C��F����Ԫ�صĻ���������Һ�����Ӧ�����ӷ���ʽ
��2��D��E��F�ļ����Ӱ뾶�ɴ�С��˳����(ֱ���û�ѧʽ��ʾ) ��
��3����Fe��D������ɵĻ�����У���������F������������Ӧˮ�����ϡ��Һ������ȫ���ܽ⡣�����õ���Һ�м������������������Һ���������ij������˳�������ϴ�ӡ�������պ�õ�һ�ֹ��壬���������ָù����������ԭ����������ǡ����ȡ���ԭ�������D���ʵ���������
Ϊ ��
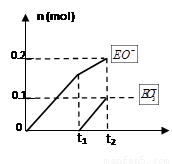
��4��һ������ʯ������ͨ��һ������E���ʣ�����ǡ����ȫ��Ӧ���������������ֺ�EԪ�ص����ӣ������������ӵ����ʵ���(n)�뷴Ӧʱ��(t)��������ͼ��ʾ����ʱ��Ӧ�Ļ�ѧ����ʽΪ ��
��5��A��B�γɵĻ�����BA���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƵĻ����д�������Ҵ���Ӧ�Ļ�ѧ����ʽ ��
��H+��HSO3����SO2��+H2O��2�֣� �� S2����Cl����Al3����2�֣� ��30����2�֣�
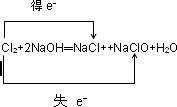
��10Cl2+10Ca(OH)2��7CaCl2+2Ca(ClO)2+Ca(ClO3)2+10H2O (2��)
��NaH+CH3CH2OH��CH3CH2ONa+H2��(2��)
��������
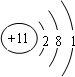
���������A�ĵ������ܶ���С�����ʣ���A����Ԫ�أ�B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���B�Ƕ�����Ԫ�أ�����B����Ԫ�أ�C��ԭ�����������������ڲ����������������C�Ƕ�����Ԫ�أ�����C����Ԫ�أ�D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����D����Ԫ�أ�B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ֣�����������Ҫ�ɷ��Ǵ������ƣ�����E����Ԫ�أ�FԪ���������������۵Ĵ�����Ϊ4����F�Ƕ�����Ԫ�أ�����F����Ԫ�ء�
��1�����־���A��B��C��F����Ԫ�صĻ���������Һ�����Ӧ�������ֻ�����Ӧ�����������ƺ����������ƣ����߷�Ӧ�����ӷ���ʽΪH+��HSO3����SO2��+H2O��
��2�����Ӻ�����Ӳ���Խ�࣬���Ӱ뾶Խ��������Ų���ͬ�����������뾶��ԭ���������������С������D��E��F�ļ����Ӱ뾶�ɴ�С��˳����S2����Cl����Al3����
��3����������ϡ���ᷴӦ��������������������������˫��ˮ����������������������������Ȼ���ټ����������������Һ��������������ƫ�����ƣ�����ϴ�Ӹ�������������ֽ��������������ù����������ԭ����������ǡ����ȣ���˵������������Ԫ�ص���������ԭ�������D���ʵ�����������ԭ�������D���ʵ�����������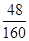 ��100%��30%��
��100%��30%��
��4������ͼ���֪������������������Ƶ����ʵ���֮�ȣ�2:1�����ڶ����ǻ�ԭ���������Ԫ�صĻ��ϼ۴�0�۷ֱ͵���1�ۺͣ�5�ۣ����Ը��ݵ��ӵĵ�ʧ�غ��֪�����������Ȼ��Ƶ����ʵ���������ơ�������Ƶ����ʵ���֮�ȣ�7:1:2����˸÷�Ӧ�����ӷ���ʽΪ10Cl2+10Ca(OH)2��7CaCl2+2Ca(ClO)2+Ca(ClO3)2+10H2O��
��5���Ҵ����ǻ���ԭ�ӵĻ����Խ�ǿ�����NaH���Ҵ���Ӧ�Ļ�ѧ����ʽΪNaH+CH3CH2OH��CH3CH2ONa+H2����
���㣺����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ���Լ�������ԭ��Ӧ���й��жϺͼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�