��Ŀ����
��11�֣��ж�����Ԫ��A��B��C��D��E����֪
�� �����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2����
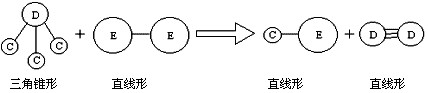
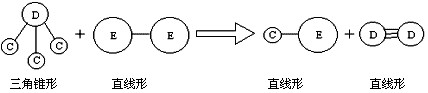
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��
��ش���������
��1�� д��AԪ�صĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4�� BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��д��ѧʽ����
��5��д��Ԫ��E�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
��1��![]() ��2��3 ����A��ԭ�Ӿ���
��2��3 ����A��ԭ�Ӿ���
��3����4��HClO4��H4SiO4����H2SiO3����5��Cl2+2OH-=Cl-+ClO-+H2O
����:

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 �� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2��