��Ŀ����
������ʳ�ι�ϵ���У�ʳ�����ϰ���������ִ����Ĺ�ũҵ�����о�����Ҫ���ã������к���Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ���ش�����ᴿ���й����⣺��1��Ϊ�˳�ȥ�����е���ɳ���ɲ�ȡ���ᴿʵ�����������������______��
��2��Ϊ�˳�ȥ���������ʣ���������ʵ�鲽������ᴿ���ټӹ���BaCl2��Һ���ڼӹ���NaOH��Һ���ۼӹ���Na2CO3��Һ���ܹ��ˣ���______���벹ȫʵ�鲽�裬���ش���۵�Ŀ����______��
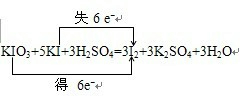
��3��2010��7��26�գ������������ˡ�ʳ���ε⺬��������������壬��������������������ʳ�����е⺬��ƽ��ˮƽ�Ĺ涨�������ͣ�ʳ�������ӵ���KIO3���������з�Ӧ������ʳ�����Ƿ�KIO3��KIO3+KI+H2SO4-I2+K2SO4+H2O ��δ��ƽ��������ƽ�÷�Ӧ����ʾ������ת�Ƶķ������Ŀ______��Ϊ�˻�ȡ��Ӧ����Һ�е�I2���ɲ�ȡ��ʵ�����������������______��
���𰸡���������1���Ȼ�����������ˮ�ģ�����ɳ��������ˮ�ģ������Һ��ķ�����Բ��ù��˷�����
��2���ڴ����ᴿʱ��Ҫ�����һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ�����̼���Ƶ�Ŀ���dz�ȥ�����ӺͶ���ı����ӣ�
��3��������ԭ��Ӧ�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ����������ݻ��ϼ۵�����������������ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ����
����⣺��1�������Ƚ����κ����е���ɳ�ܽ���ˮ���Ȼ�����������ˮ�ģ�����ɳ��������ˮ�ģ��������Һ��ķ�����Բ��ù��˷������ʴ�Ϊ���ܽ⡢���ˣ�
��2���ڴ����ᴿʱ��Ҫ�����һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ�����̼���Ƶ�Ŀ���dz�ȥ�����ӺͶ���ı����ӣ��ʴ�Ϊ����������� ��ȥCa2+������Ba2+��
��3�����ݵ����غ��ԭ���غ�����ƽ����ʽ����������ԭ��ӦKIO3+5KI+3H2SO4=3I2+3K2SO4+3H2O�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=6������ת��������£� ���ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ�����������Ȼ�̼����ȡ��Ȼ��������Ȼ�̼�͵�ķе�IJ�ͬ�����룬
���ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ�����������Ȼ�̼����ȡ��Ȼ��������Ȼ�̼�͵�ķе�IJ�ͬ�����룬
�ʴ�Ϊ�� ����ȡ������
����ȡ������
���������⿼��ѧ���йش��ε��ᴿ�Լ�������ԭ��Ӧ�еĵ���ת��֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���
��2���ڴ����ᴿʱ��Ҫ�����һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ�����̼���Ƶ�Ŀ���dz�ȥ�����ӺͶ���ı����ӣ�
��3��������ԭ��Ӧ�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ����������ݻ��ϼ۵�����������������ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ����
����⣺��1�������Ƚ����κ����е���ɳ�ܽ���ˮ���Ȼ�����������ˮ�ģ�����ɳ��������ˮ�ģ��������Һ��ķ�����Բ��ù��˷������ʴ�Ϊ���ܽ⡢���ˣ�
��2���ڴ����ᴿʱ��Ҫ�����һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ�����̼���Ƶ�Ŀ���dz�ȥ�����ӺͶ���ı����ӣ��ʴ�Ϊ����������� ��ȥCa2+������Ba2+��
��3�����ݵ����غ��ԭ���غ�����ƽ����ʽ����������ԭ��ӦKIO3+5KI+3H2SO4=3I2+3K2SO4+3H2O�У����ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=6������ת��������£�
 ���ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ�����������Ȼ�̼����ȡ��Ȼ��������Ȼ�̼�͵�ķе�IJ�ͬ�����룬
���ⵥ�ʴӵ�ˮ����ȡ���Բ�����ȡ��Һ�����������Ȼ�̼����ȡ��Ȼ��������Ȼ�̼�͵�ķе�IJ�ͬ�����룬�ʴ�Ϊ��
 ����ȡ������
����ȡ���������������⿼��ѧ���йش��ε��ᴿ�Լ�������ԭ��Ӧ�еĵ���ת��֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ