��Ŀ����
��(Ti)����Ϊ��������֮��ĵ���������Ҳ����˵21�������ѵ����͡����ڵؿ��еĺ��������٣����ѵ�ұ��������δ���ͻ�ƣ�Ŀǰ��ֻ���ڼ������
����ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ���ɴ�������Դ�����ʣ����ٻ�����Ⱦ��
����д���пհף�
��1���ö��Ե缫���2 Lʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ_______________________________���������ϲ���224 mL���壨��״����ʱ��������Һ��pH= ��������ǰ����Һ�������,ʳ��ˮ��������
��2��д���������������Ȼ��õ����Ȼ��ѵĻ�ѧ����ʽ ������ʾ��FeTiO3��TiΪ+4�ۣ�
��3����Ӧ2Mg��TiCl4 2MgCl4��Ti��Ar�����н��е�������____________________��
2MgCl4��Ti��Ar������������____________________��
��4����������һ����Ҫ�����ȼ�ϣ�����ͨ���״����Ӽ���ˮ�Ƶã�
2CH3OH(g) CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
T1 ��ʱ���ں����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯����ͼ��ʾ��
��T1 ��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ������������
����ͬ�����£����ı���ʼŨ�ȣ�ijʱ�̸����Ũ������Ϊc(CH3OH)="0.4" mol/L��c(H2O)="0.6" mol/L��(CH3OCH3)="1.2" mol/L����ʱ�����淴Ӧ���ʵĴ�С��v��������v��(�>������<����=��)��
��5����������ҵ���У��ϳ�192�ּ״�����������ⲹ��H2__________�� ���������������������ʵ��κ���ʧ����
��14�֣�ÿ��2��) ��1��2Cl����2H2O 2OH����H2����Cl2����12
2OH����H2����Cl2����12
��2��2FeTiO3��6C��7Cl2 2TiCl4��2FeCl3��6CO��
2TiCl4��2FeCl3��6CO��
��3����ֹ������Mg��Ti������е�O2����CO2��N2����Ӧ�� ��4�� ��5 �ڣ� ��5��10
���������������1���ö��Ե缫���ʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ��2Cl����2H2O 2OH����H2����Cl2���������������ӷŵ磬�������������������ʵ�����0.224L��22.4L/mol��0.01mol������ݷ���ʽ��֪�����ɵ�����������0.02mol����������������Һ��Ũ����0.02mol��2L��0.01mol/L����pH��12��
2OH����H2����Cl2���������������ӷŵ磬�������������������ʵ�����0.224L��22.4L/mol��0.01mol������ݷ���ʽ��֪�����ɵ�����������0.02mol����������������Һ��Ũ����0.02mol��2L��0.01mol/L����pH��12��
��2��������̼������������CO�������������Ȼ��õ����Ȼ��ѵĻ�ѧ����ʽ��2FeTiO3��6C��7Cl2 2TiCl4��2FeCl3��6CO��
2TiCl4��2FeCl3��6CO��
��3���ڸ�����þ���Ѿ��ܺͿ����е�����������CO2��Ӧ�����Է�Ӧ��Ar�����н��е������Ƿ�ֹ������Mg��Ti������е�O2����CO2��N2����Ӧ��
��4���ٻ�ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը���ͼ���֪ƽ��ʱ���ѡ�ˮ�����ͼ״���Ũ�ȷֱ��ǣ�mol/L��1��0.8��0.4����÷�Ӧ��ƽ�ⳣ��K��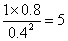 ��
��
��ijʱ�̸����Ũ������Ϊc(CH3OH)="0.4" mol/L��c(H2O)="0.6" mol/L��(CH3OCH3)="1.2" mol/L����ʱ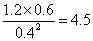 ��5����������Ӧ���ʴ����淴Ӧ���ʡ�
��5����������Ӧ���ʴ����淴Ӧ���ʡ�
��5�������ɷ���ʽCO��g��+2H2��g�� CH3OH��g����2FeTiO3+6C+7Cl2��2FeCl3+2TiCl4+6CO��2NaCl+2H2O��2NaOH+H2��+Cl2���ɵ����¹�ϵʽ��6CH3OH��6CO��7Cl2��7H2����6CH3OH��12H2����ÿ����6molCH3OH��192g������ⲹ��5molH2��10g����������192t�״�����������ⲹ��10t������
CH3OH��g����2FeTiO3+6C+7Cl2��2FeCl3+2TiCl4+6CO��2NaCl+2H2O��2NaOH+H2��+Cl2���ɵ����¹�ϵʽ��6CH3OH��6CO��7Cl2��7H2����6CH3OH��12H2����ÿ����6molCH3OH��192g������ⲹ��5molH2��10g����������192t�״�����������ⲹ��10t������
���㣺�����ⱥ��ʳ��ˮ���жϺͼ��㡢������ԭ��Ӧ����ʽ����д����Ӧ�����Ŀ��ơ�ƽ�ⳣ���ļ����Ӧ���Լ�����ͼ���йؼ���
�����������Ǹ߿��еij������ͣ��Ѷȴ��ۺ���ǿ����ѧ����Ҫ��ߡ�������ע�ضԻ���֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�����������������ѧ����ѧ����������������Ҫ��ѧ���ܹ��߱�ͨ������Ȼ�硢����������Ϳ�ѧʵ���л�ѧ�����Լ����ģ�͡�ͼ�κ�ͼ���ȵĹ۲죬��ȡ�йصĸ���֪ʶ��ӡ�����÷������Ƚϡ����������ɵȷ���������ȡ����Ϣ���г����ӹ���Ӧ�õ��������ܹ����ݡ�ȷ�ػ�ȡ���������������Ϣ����������֪ʶ���ϣ��ڷ������۵Ļ�����Ӧ������Ϣ��������

 ��У����ϵ�д�
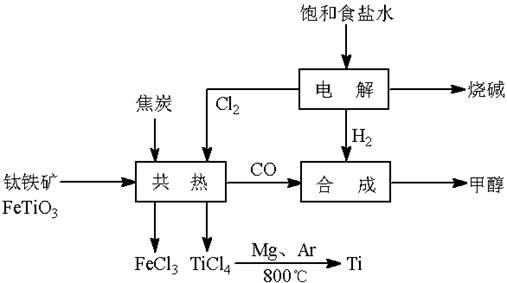
��У����ϵ�д�(13��) ��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
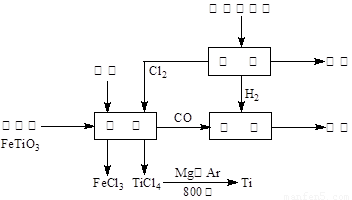
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti(s)����Ar�����н��е�������
��
2MgCl2(s) + Ti(s)����Ar������������
��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��


 2MgCl4��Ti��Ar������������____________________��
2MgCl4��Ti��Ar������������____________________�� CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol


 2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
�� ��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��