��Ŀ����
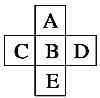
����Ŀ��ͼ1�Dz��ֶ�����Ԫ�صij������ϼ���ԭ�������Ĺ�ϵͼ��

��ش��������⣺
(1)Ԫ��F�����ڱ��е�λ��Ϊ________________
(2)C��D��E��G�ļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________(�����ӷ��ű�ʾ)��
(3)��Ԫ������X�Ǻ���Ԫ��A��18���ӷ��ӣ�3 g X(g)��25 �� 101 kPa ����ȫȼ�������ȶ��Ļ�����ʱ�ų�Q kJ��������д����ʾXȼ���ȵ��Ȼ�ѧ����ʽ��________________
(4)ijͬѧ���ʵ����ͼ2��ʾװ��֤��Ԫ��A��B��F�ķǽ�����ǿ��(������Һb����Һc������)��
����ҺbΪ_________________
����Һc�з�����Ӧ�����ӷ���ʽΪ__________________
���𰸡��������ڵڢ�A�� S2��>O2��>Na��>Al3�� C2H6(g)��7/2O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol ����NaHCO3��Һ SiO32-��CO2��H2O===H2SiO3����CO32-
��������
���������֪�����⿼��Ԫ�����ڱ���Ԫ�ػ��ϼۡ����Ӱ뾶��С���Ȼ�ѧ����ʽ����д������Ԫ�������ɡ����Ӱ뾶��С�ȽϷ������Ȼ�ѧ����ʽ��д���������
��1����ͼ1�����ɵã�AΪC��BΪN��CΪO��DΪNa��EΪAl��FΪSi��GΪS,���F�����ڱ��е�λ��Ϊ�������ڵ���A�壻
�ʴ�Ϊ���������ڵ���A�壻
��2�����Ӳ�Խ�࣬���Ӱ��Խ������ͬ�����Ų���������ԭ������������Ӱ뾶С����S2��>O2��>Na��>Al3����
�ʴ�Ϊ��S2��>O2��>Na��>Al3����
(3) ��Ԫ������X�Ǻ���Ԫ��A��18���ӷ��ӣ�XΪC2H6��3 g X(g)��25 �� 101 kPa ����ȫȼ�������ȶ��Ļ�����ʱ�ų�Q kJ����������Xȼ���ȵ��Ȼ�ѧ����ʽΪC2H6(g)��![]() O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
�ʴ�Ϊ��C2H6(g)��![]() O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
O2(g)===2CO2(g)��3H2O(l) ��H����10QkJ/mol��
��4����֤��Ԫ��A��B��F�ķǽ�����ǿ������Ӧ��A��B��F��Ӧ������������ˮ���������Ӧ�ν��з�Ӧ����֤�������Һa��b��c�ֱ�ΪHNO3������NaHCO3��Na2SiO3��Һ��
�ʴ�Ϊ������NaHCO3��Һ��
��Һb�в����Ķ�����̼ͨ��c�� Na2SiO3��Һ�з�����Ӧ�����ӷ���ʽΪSiO32-��CO2��H2O===H2SiO3����CO32-��
�ʴ�Ϊ��SiO32-��CO2��H2O===H2SiO3����CO32-��

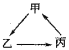
����Ŀ�����и��������У����ܰ���ͼ��ʾ![]() ��
��![]() ����ʾһ�����
����ʾһ�����![]() ��ϵ�ת�����ǣ� ��
��ϵ�ת�����ǣ� ��
A | B | C | D | |
�� | Al | Na | SO2 | Cu |
�� | NaAlO2 | NaOH | SO3 | CuO |
�� | Al(OH)3 | NaCl | H2SO4 | CuCl2 |
A.AB.BC.CD.D
����Ŀ��ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1��������к��ȵIJⶨ��
������0.50 mol��L-1 NaOH��Һ
(1)��ʵ���д�ԼҪʹ��245 mL NaOH��Һ,������Ҫ����NaOH����_____g��
(2)���±���ѡ��,����NaOH��������Ҫ��������(����ĸ)________��
���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
���� |
|
|
|
|
|
|
��� | a | b | c | d | e | f |
�ⶨϡ�����ϡ����������Һ�к��ȵ�ʵ��װ����ͼ��ʾ��

(1)������1 mol H2Oʱ��Ӧ�ų�������Ϊ57.3 kJ,д���÷�Ӧ���Ȼ�ѧ����ʽ:__________��
(2)ȡ50 mL NaOH��Һ��30 mL�������ʵ��,ʵ���������±���
������д�±��еĿհ�:
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ(t2-t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
��������ʵ����ֵ������Ϊ____kJmol-1,��57��3 kJmol-1��ƫ��,����ƫ���ԭ�������_____(����ĸ)��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
����Ŀ������ʵ����̲��ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ʵ��Ŀ�� | ʵ����� | |
A | ̽��ά����C�Ļ�ԭ�� | ��ʢ��2mL��ɫ�Ȼ�����Һ���Թ��еμ�Ũ��ά����C��Һ���۲���ɫ�仯 |
B | ����100mL1.0mol/L CuSO4��Һ | ��25.0gCuSO4��5H2O���100mL��Һ |
C | ��֤X��Һ���Ƿ���Fe2+ | ��X��Һ�еμӼ���������ˮ�����ټ�������KSCN��Һ���۲���Һ��ɫ�仯 |
D | ��ȥ����KNO3��������NaCl | ��������Ƴ��ȵı�����Һ����ȴ�ᾧ�����ˡ�ϴ�ӡ����� |
A.AB.BC.CD.D