��Ŀ����
 ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У���1����BΪCaCO3��CΪC6H5ONa��Һ��ʵ��۲쵽С�Թ�����Һ����ǣ�����A��̼�������
ǿ
ǿ
���ǿ��������������Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е���������ɫ���DZ����
��ɫ���DZ����
����2����A��Ũ��ˮ��B����ʯ�ң�ʵ���й۲쵽C��Һ���γɳ�����Ȼ������ܽ⣬����Һǡ�ó���ʱ���ر�E��Ȼ�����ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������C����������
AgNO3
AgNO3
��д��ѧʽ���Ļ��Һ����������Ӧ�Ļ�ѧ����ʽΪCH2OH��CHOH��4CHO+2Ag��NH3��2OH
H2O+2Ag��+3NH3+CH2OH��CHOH��4COONH4
| �� |
CH2OH��CHOH��4CHO+2Ag��NH3��2OH
H2O+2Ag��+3NH3+CH2OH��CHOH��4COONH4
������D�ڴ�ʵ���е�������| �� |
��ֹ����
��ֹ����
����������1�����ݽ�ǿ��Ŀ���ȡ�������ԭ���ƶϳ�A�����ʣ����ݱ��ӵ��ܽ����жϣ�
��2��������ܷ���������Ӧ֪��CΪ�����Ǻ�����������������ǵ����ʽ��
��2��������ܷ���������Ӧ֪��CΪ�����Ǻ�����������������ǵ����ʽ��
����⣺��1���۲쵽С�Թ�����Һ����ǣ�˵�����ɶ�����̼����A������Ӧ��̼�������ǿ�����ɱ�����������ˮ��������Һ����壬
�ʴ�Ϊ��ǿ����ɫ���DZ���壻
��2���Թܱڳ��ֹ�����������˵�����ɰ���������Ũ��ˮ����ε�Ũ��Һ����ʯ�ҷ�Ӧ��ȡ��C��ӦΪ��������������Һ�ķ�Ӧ����C��ӦΪAgNO3�������ǵĻ��Һ������ʱ������CH2OH��CHOH��4CHO+2Ag��NH3��2OH
H2O+2Ag��+3NH3+CH2OH��CHOH��4COONH4��װ��D����ֹ���������ã�
�ʴ�Ϊ��AgNO3��CH2OH��CHOH��4CHO+2Ag��NH3��2OH
H2O+2Ag��+3NH3+CH2OH��CHOH��4COONH4����ֹ������
�ʴ�Ϊ��ǿ����ɫ���DZ���壻
��2���Թܱڳ��ֹ�����������˵�����ɰ���������Ũ��ˮ����ε�Ũ��Һ����ʯ�ҷ�Ӧ��ȡ��C��ӦΪ��������������Һ�ķ�Ӧ����C��ӦΪAgNO3�������ǵĻ��Һ������ʱ������CH2OH��CHOH��4CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��AgNO3��CH2OH��CHOH��4CHO+2Ag��NH3��2OH
| �� |
����������Ϊһʵ���ۺ��⣬�����˳�����Ҫ�������л�ʵ�飮������ƱȽ���ӱ����֪ʶ��Ƚϴ�1����Ҫ���鳣���������ǿ�����ɣ���2������Ϣ����������ֻҪץס��һ�㣬���ܽ������뵽������Һ��

��ϰ��ϵ�д�
�����Ŀ
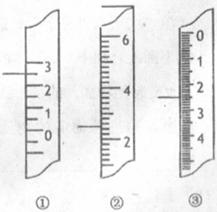 (4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ�
(4��)��(1)�¶ȼƳ�����������ƻ�ѧʵ����¶ȡ� C�����ǵζ��ܣ�����Ϊ3.5 mL
C�����ǵζ��ܣ�����Ϊ3.5 mL �ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
�ֽ�6.0molCO2��8.0molH2����2L���ܱ������У����H2�����ʵ�����n����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
 ���Դ˵������Դ����ʵ������ģ������Ʒ����
���Դ˵������Դ����ʵ������ģ������Ʒ����