��Ŀ����
13��ʪ����п��ұ�����̿�����ͼ1���Ա�ʾ��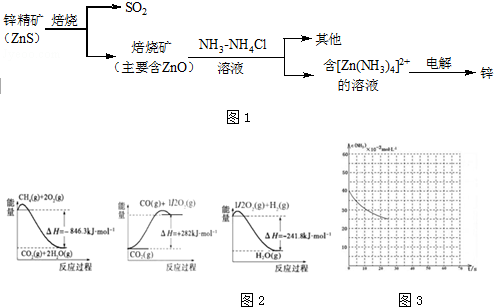
��ش��������⣺
��1����֪ZnO�������������д��ZnO��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��2NaOH+ZnO=Na2ZnO2+H2O��
��2������������������п�ĵ缫��ӦʽΪ��[Zn��NH3��4]2++2e-=Zn+4NH3����
��3��������SO2����Ba��NO3��2��Һ���գ����ֲ������Ϊ��ҵԭ�ϣ��䷴Ӧ�����ӷ���ʽΪ��3SO2+2H2O+2NO3-+3Ba2+=3BaSO4��+2NO��+4H+��
��4����������Ҫ�Ļ�����Ʒ֮һ���ϳɰ��õ��������Լ���Ϊԭ���Ƶã�CH4��g��+H2O��g��?CO��g��+3H2��g����
�йػ�ѧ��Ӧ�������仯��ͼ2��ʾ����CH4��g����H2O��g����Ӧ����CO��g����H2��g�����Ȼ�ѧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+161.1kJ•mol-1��
��5��CO�Ժϳɰ��Ĵ����ж������ã��������������ͭ��Һ������ԭ������CO���䷴Ӧԭ��Ϊ��
[Cu��NH3��2CH3COO]��l��+CO��g��+NH3��g��?[Cu��NH3��3]CH3COO•CO��l����H��0������CO�������ͭ��Һ�����ʵ��������ֿ��������ָ�������CO�������Թ�ѭ��ʹ�ã����������������ǣ�B����дѡ���ţ�
A�����¡���ѹ B�����¡���ѹ C�����¡���ѹ D�����¡���ѹ
��6��ij�¶��£����ݻ�Ϊ100L���ܱ�������ͨ��4mol NH3��2molCO2������2NH3��g��+CO2��g��?CO��NH2��2��l��+H2O��g����Ӧ���÷�Ӧ���е�40sʱ�ﵽƽ�⣬��ʱCO2��ת����Ϊ50%��ͼ3�е����߱�ʾ�÷�Ӧ��ǰ25s�ڵķ�Ӧ�����е�NH3Ũ�ȱ仯������Ӧ������70s����������������������£�����ͼ����ʵ����ʹ�ô���ʱ�÷�Ӧ�ӿ�ʼ��ƽ��ʱ�����ߣ�
���� п�����յõ����������ZnO����ZnO����NH3-NH4Cl��Һ�У�������Ӧ�õ�[Zn��NH3��4]Cl2�����[Zn��NH3��4]Cl2��Һ�õ�Zn��
��1��ZnO�������������ZnO��NaOH��Һ��Ӧ����Na2ZnO2��
��2��������������п�ĵ缫��[Zn��NH3��4]2+ �õ��ӷ�����ԭ��Ӧ��
��3��SO2����Ba��NO3��2��Һ���գ�����������ԭ��Ӧ����һ�����������ᱵ���ݴ�д���ӷ���ʽ��
��4����ͼ2��֪��CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-846.3KJ/mol ��
CO2��g��=CO��g��+$\frac{1}{2}$O2��g����H=+282KJ/mol ��
H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8KJ/mol ��
����Ȼ�ѧ����ʽ��˹���ɿ�֪��+��-�ۡ�3�õ������Ȼ�ѧ����ʽ��
��5������ƽ���ƶ���������ж���Ҫ��������
��6�����ݴ���ֻ�ӿ췴Ӧ���ʲ�Ӱ��ƽ��Ũ�Ȼ���ͼ��
��� �⣺��1��ZnO�������������ZnO��NaOH��Һ��Ӧ����ƫп���ƺ�ˮ����Ӧ����ʽΪ2NaOH+ZnO=Na2ZnO2+H2O��
�ʴ�Ϊ��2NaOH+ZnO=Na2ZnO2+H2O��
��2��������������п�ĵ缫��[Zn��NH3��4]2+ �õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ[Zn��NH3��4]2++2e-=Zn+4NH3����
�ʴ�Ϊ��[Zn��NH3��4]2++2e-=Zn+4NH3����
��3��SO2����Ba��NO3��2��Һ���գ�����������ԭ��Ӧ����һ�����������ᱵ����Ӧ�����ӷ���ʽΪ3SO2+2H2O+2NO3-+3Ba2+=3BaSO4��+2NO��+4H+��
�ʴ�Ϊ��3SO2+2H2O+2NO3-+3Ba2+=3BaSO4��+2NO��+4H+��
��4����ͼ2��֪��CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-846.3KJ/mol��
CO2��g��=CO��g��+$\frac{1}{2}$O2��g����H=+282KJ/mol��
H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8KJ/mol��
����Ȼ�ѧ����ʽ��˹���ɿ�֪��+��-�ۡ�3�ɵ������Ȼ�ѧ����ʽ����CH4��g��+H2O��g��=CO��g��+3H2��g����H=��-846.3KJ/mol��+��+282KJ/mol ��-��-241.8KJ/mol ����3=+161.1 kJ•mol-1������CH4��g����H2O��g����Ӧ����CO��g����H2��g�����Ȼ�ѧ����ʽΪ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+161.1 kJ•mol-1��
�ʴ�Ϊ��CH4��g��+H2O��g��=CO��g��+3H2��g����H=+161.1 kJ•mol-1��
��5������CO�������ͭ��Һ�����ʵ��������ֿ��������ָ�������CO�������Թ�ѭ��ʹ�ã����ݻ�ѧƽ��[Cu��NH3��2CH3COO]��l��+CO��g��+NH3��g��?[Cu��NH3��3]CH3COO•CO��l������H��0����Ӧ�����������С�ķ��ȷ�Ӧ��ƽ�����������������ԭ�������������������Ǹ��µ�ѹ��
�ʴ�Ϊ��B��
��6������Ӧ������70s����������������������£�ʹ�ô���ʱ���ı䷴Ӧ���ʣ����ı仯ѧƽ�⣬���ߵ�ת�۵��ں�����40֮ǰ�������������20�����ϣ��÷�Ӧ�Ľ�������Ϊ��ͼ��ʾ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼����������ᴿ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ��漰�Ȼ�ѧ����ʽ��д����ѧƽ��Ӱ�����ء�ƽ����㡢���ȣ�������ط�Ӧԭ��Ϊ���Ĺؼ������ط�����Ӧ���������ۺϿ��飮

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д���1����ҵ����������������ɲ������·�Ӧ��CH4��g��+2NO2��g��?N2��g��+CO2��g��+2H2O��g����H
���¶�ΪT1��T2ʱ���ֱ�0.5mol CH4��1.2mol NO2�������Ϊ1L���ܱ������У����NO2�����ʵ�����ʱ��仯�������±���
�¶�/�� ʱ��/min | 0 | 10 | 20 | 40 | 50 |
| T1 | 1.2 | 0.9 | 0.7 | 0.4 | 0.4 |
| T2 | 1.2 | 0.8 | 0.56 | �� | 0.5 |
��T1��T2��������������¿�ͬ������H��0���ж������������¶ȣ�NO2�����ʵ�������ƽ�������ƶ�������ӦΪ���ȷ�Ӧ��
�۷�ӦCH4��g��+2NO2��g��?N2��g��+CO2��g��+2H2O��g����ƽ�ⳣ������ʽK=$\frac{c��{N}_{2}����c��C{O}_{2}����{c}^{2}��{H}_{2}O��}{c��C{H}_{4}����{c}^{2}��N{O}_{2}��}$���¶�ΪT1��ʱ��K��ֵΪ6.4��
���¶�ΪT2��ʱ����ƽ������������м���0.5mol CH4��1.2mol NO2������ƽ��ʱCH4��ת���ʽ���С�����������С�����䡱����
��2����֪��ӦN2O4��g��?2NO2��g�������¶����ߣ�����������ɫ�����һ����N2O4���������ͼ��������һ��ʱ���ѹ�������������������������ʣ�������ɫԽdz������Խ����ʱ��仯��ͼ��ʾ��
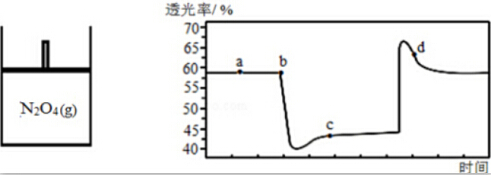
��a����c����ȣ�c ��NO2���������c����ϵ�¶ȸ��͵���a��
��d�㣬v����NO2����v����NO2�������������������=������
������˵����˵�����ʲ��ٷ����ı����ad��
a��������ɫ���ٸı� b����H���ٸı�
c��v����N2O4��=2v����NO2�� d��N2O4��ת���ʲ��ٸı䣮
| �� | �� | �� | �� | |
| pH | 11 | 11 | 3 | 3 |
| ��Һ | ��ˮ | NaOH��Һ | ���� | ���� |
| A�� | �ۢ��зֱ�������������ƾ��壬����ҺpH������ | |
| B�� | �ڢ�����Һ�������ϣ�������Һ�У�c��OH-����c��H+�� | |
| C�� | �ֱ��ˮϡ��10������Һ��pH���٣��ڣ��ܣ��� | |
| D�� | V1L�ܺ�V2L����Һ��Ϻ���pH=7����V1��V2 |
| A�� | ��������ɹ㷺����ʳƷ������ | |
| B�� | ����ϩ������Ʒ������ʳƷ�İ�װ | |
| C�� | ����������Һ�����ڻ���������ɱ�� | |
| D�� | ��������ұ����������ԭ�ϣ�Ҳ��һ�ֽϺõ��ͻ���� |
| A�� | ��NaAlO2��Һ��ͨ�����CO2��AlO2-+CO2+2H2O�TAl��OH��3��+HCO3- | |
| B�� | ��������Һ�������Һ��ϣ�SiO32-+2H+�TH2SiO3�� | |
| C�� | MnO2��Ũ���ᷴӦ��Cl2��MnO2+4HCl$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++2Cl-+Cl2��+2H2O | |
| D�� | Ũ�����м���������۲����ȣ�Fe+3NO3-+6H+$\frac{\underline{\;\;��\;\;}}{\;}$Fe3++3NO2��+3H2O |
| ���� �� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | N | O | F | Ne | ||||
| 3 | Na | Mg | Al | Si | S | Cl |
��2��Neԭ�ӽṹʾ��ͼΪ
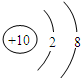 ��
����3��N��O�У�ԭ�Ӱ뾶��С����O�� ��4��H2S��HCl�У����ȶ��Խ�ǿ����HCl��
��5��MgO��Al2O3�������������������Al2O3��
��6��Ԫ������������Ӧ��ˮ�����У�������ǿ����NaOH���ѧʽ����
��7��SiO2������������ά����һ�ָ����ܵ��ִ�ͨѶ���ϵ����ƣ���
��8������Ϊ����ɫ���������Ĺ������⣬���Ļ�ѧʽ��H2O2��������Ԫ������Ԫ�ص�������Ϊm��H����m��O��=1��16�������ԭ��������H-1��O-16��
��9��þ�������������ɻ����������Ҫ���ϣ�д����ҵ�ϵ�������Ȼ�þ��ý���þ�Ļ�ѧ����ʽ��MgCl2�����ڣ�$\frac{\underline{\;ͨ��\;}}{\;}$Mg+Cl2��
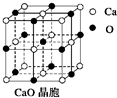 ��Ҫ��ش��������⣺
��Ҫ��ش��������⣺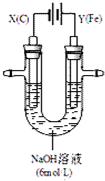 ��1�����ڹ�����ռ����Ҫ��λ��
��1�����ڹ�����ռ����Ҫ��λ��