��Ŀ����
16����ѧ���ı仯�����ʵı仯�йأ���1������4���仯�У���NaCl ����ˮ ��HCl ����ˮ ��������������ȼ������ˮ ������������ȼ�������Ȼ��ƣ������й��ۼ����ƻ��ı仯�Ǣڢۢܣ��� ����ţ�
��2��±��X2��F2��Cl2��Br2��I2��������H2��Ӧ����HX����ԭ�ӽṹ����ԭ��±��ԭ�ӵ�������������Ϊ7��
��3��HX�ĵ���ʽ��
 �����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳����HF��HCl��HBr��HI��
�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ��HX���ۼ��ļ�����ǿ������˳����HF��HCl��HBr��HI����4������±��ԭ�Ӻ˵���������ӣ����й���±�ص�˵����ȷ����abd��ѡ����ĸ����
a��X2����ɫ���� b��X2��H2��Ӧ�ľ��ҳ̶�����
c��HX�Ļ�ԭ������ d��HX���ȶ�������
��5����֪��1mol H-H����1mol I-I��1mol H-I���ֱ���Ҫ���յ�����Ϊ436kJ��151kJ��299kJ�����������͵ⷴӦ����1mol HI��Ҫ�ų�����ų��������ա���5.5kJ��������
���� ��1����NaCl ����ˮ���ƻ����Ӽ��� ��HCl ����ˮ�ƻ����ۼ��� ��������������ȼ������ˮ�ƻ����ۼ��� ������������ȼ�������Ȼ����ƻ����������ۼ���
��2��±��ԭ�ӵ�������������Ϊ7��ֻҪ�γ�һ�Թ��õ��Ӷԣ����ȶ��ṹ��
��3��HX�ĵ���ʽ��  �����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ���ǽ�����Խǿ�������õ��Ӷ�����Խǿ��
�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ���ǽ�����Խǿ�������õ��Ӷ�����Խǿ��
��4��±�ص��ʴӷ������⣬���ʵ���ɫ����������������ܶ���������ǽ��������������Ӧ���⻯����ȶ��������� ��5���ɼ�����Ҫ�����������¼�����Ҫ�ͷ����������ɼ��������յ����������¼������ͷŵ�����ʱ����ӦΪ���ȷ�Ӧ����֮��Ϊ���ȷ�Ӧ��
��� �⣺��1����NaCl ����ˮ���ƻ����Ӽ��� ��HCl ����ˮ�ƻ����ۼ��� ��������������ȼ������ˮ�ƻ����ۼ��� ������������ȼ�������Ȼ����ƻ����������ۼ��������й��ۼ����ƻ��ı仯�Ǣڢۢܣ��ʴ�Ϊ���ڢۢܣ�
��2��±��ԭ�ӵ�������������Ϊ7��ֻҪ�γ�һ�Թ��õ��Ӷԣ����ȶ��ṹ���ʴ�Ϊ��±��ԭ�ӵ�������������Ϊ7��
��3��HX�ĵ���ʽ��  �����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ���ǽ�����Խǿ�������õ��Ӷ�����Խǿ������HX���ۼ��ļ�����ǿ������˳����HF��HCl��HBr��HI���ʴ�Ϊ��
�����ۼ��ļ����湲�õ��Ӷ�ƫ�Ƴ̶ȵ��������ǿ���ǽ�����Խǿ�������õ��Ӷ�����Խǿ������HX���ۼ��ļ�����ǿ������˳����HF��HCl��HBr��HI���ʴ�Ϊ�� �� HF��HCl��HBr��HI��
�� HF��HCl��HBr��HI��
��4��a��F2��Cl2��Br2��I2���ʵ���ɫ�ֱ���dz����ɫ������ɫ�������ɫ���Ϻ�ɫ��������ɫ�������ȷ��
b��F2��Cl2��Br2��I2���ʵ������Լ�����H2��Ӧ�ľ��ҳ̶�����������ȷ��
c��HX�Ļ�ԭ������ǿ���ʴ���
d������F��Cl��Br��I��˳�˵���������ӣ����ǵ��⻯����ȶ�������������ȷ��
��ѡ��abd��
��5�������͵ⷴӦ����2molHI�ǣ��ɼ���������������ֵΪ��436kJ+151kJ=587KJ���¼������ͷ�����Ϊ��299kJ��2=598KJ���ɼ��������յ�����С���¼������ͷŵ���������ӦΪ���ȷ�Ӧ���ų�������Ϊ��598KJ-587KJ=11KJ����������1mol HI��Ҫ�ų�������Ϊ��5.5KJ���ʴ�Ϊ���ų��� 5.5��
���� ������Ҫ�����˻�ѧ���ͼ����йص�֪ʶ�����վɼ�����Ҫ�����������¼�����Ҫ�ͷ������ǽ��Ĺؼ�����Ŀ�ѶȲ���

| A�� | ����Ԫ��ԭ�ӵĵ��Ӳ���ȫ������s���� | |
| B�� | ���������Ų�Ϊ2s22p6��ԭ�Ӻ����������Ų�Ϊ2s22p6������ | |
| C�� | 3p�ܼ���ֻ��1���չ����ԭ�Ӻ�3p�ܼ���ֻ��1��δ�ɶԵ��ӵ�ԭ�� | |
| D�� | M���ϵ�s��p�ܼ��϶������˵��Ӷ�d���δ�ŵ��ӵ�����ԭ�� |
| A�� | �Ž��з����� | B�� | Ŧ���ȣ�Ӣ���� | C�� | ��Ү�����¹��� | D�� | �������������� |
| A�� | ���ԭ�ӽṹʾ��ͼ�� | |
| B�� | Be2+�����е��������͵�����֮��Ϊ2��1 | |
| C�� | ԭ�Ӻ�����8�����ӵ�̼ԭ�ӣ�${\;}_{8}^{14}$C | |
| D�� | ͬһԪ�صĸ���ͬλ�ص��������ʡ���ѧ���ʾ���ͬ |
| A�� | v��O2��=0.01mol/��L•s�� | B�� | v��NO��=0.008mol/��L•s�� | ||
| C�� | v��H2O��=0.001mol/��L•s�� | D�� | v��NH3��=0.002mol/��L•s�� |
 ��
����2����Ҫ��д���ɵڶ�����Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӵĻ�ѧʽ������дһ�֣������������CH4��CF4�������η���NH3��NF3��V�η���H2O��
��3����Ҫ����д�±�������ŵĿո�
| Ԫ�ط��� | �����Ų�ʽ | �۲�����Ų� | �����ڱ��е�λ�� |
| �� | 1s22s22p6 | �� | �� |
| Cr | �� | �� | �� |
| A�� | 0.1 mol/L������15mL | B�� | 0.15 mol/L ��������Һ8mL | ||
| C�� | 0.2 mol/L ������12mL | D�� | 18 mol/L��Ũ����15mL |
| A�� | FeCl2 | B�� | H2SiO3 | C�� | NH4NO3 | D�� | HCl |
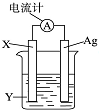 ����������ԭ��Ӧ��2Ag++Cu�TCu2++2Ag��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺
����������ԭ��Ӧ��2Ag++Cu�TCu2++2Ag��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺