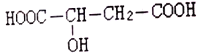��Ŀ����
����Ŀ��ȼú����������������Ŀǰ�о����ȵ㡣
��1����CH4����ԭ��������������������������Ⱦ����֪:
��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574 kJmol-1
��CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160 kJmol-1
��H2O(g)=H2O(l) ��H=-44 kJmol-1
д��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O(l) ���Ȼ�ѧ����ʽ_________��
��2��ij����С���о���������--�����շ�ͬʱ�ѳ�SO2��NO���գ��������̷�Ӧԭ������Ӧ�ȡ�����������£�
��Ӧ��NO(g)+ O3(g) NO2(g)+O2(g) ��H1= -200.9 kJmol-1Ea1= 3.2 kJmol-1
��Ӧ��SO2(g)+ O3(g) SO3(g)+O2(g)��H2= -241.6 kJmol-1 Ea2= 58 kJmol-1
��֪����ϵ�г��������ֽⷴӦ��2O3(g) 3O2(g)����ش�:
�����������䣬ÿ�����ݻ�Ϊ2L�ķ�Ӧ���г��뺬1.0 mol NO��1.0 mol SO2��ģ��������2.0 mol O3���ı��¶ȣ���Ӧ��ͬʱ��t����ϵ��NO��SO2��ת������ͼ��ʾ��

����ͼ��֪��ͬ�¶���NO��ת����Զ����SO2������������ݷ��������ԭ��_______��
������˵����ȷ���� ____________ ��
A��P��һ��Ϊƽ��״̬��
B���¶ȸ���200���NO��SO2��ת�������¶����������½������Ϊ��
C�������������䣬����С��Ӧ�����ݻ������NO��SO2��ת����
�ۼ���100��ʱP��NOת����Ϊ85%����Q��SO2ת����Ϊ30%����Ϊƽ��㣬��ʱ��Ӧʱ��Ϊ10���ӣ������ֽⷴӦ�ij���ռ�������������10%������ϵ��ʣ��O3�����ʵ�����_______mol��NO��ƽ����Ӧ����Ϊ_______________����Ӧ���ڴ�ʱ��ƽ�ⳣ��Ϊ_______________ ��
��3���õ绯ѧ��ģ�ҵ����SO2�������Ṥҵβ���е�SO2ͨ����ͼװ��(�缫��Ϊ���Բ���)����ʵ�飬�������Ʊ����ᣬͬʱ��õ��ܣ�

��M�������ĵ缫��ӦʽΪ ____________��
�ڵ����·ͨ��0.2 mol����ʱ�����ӽ���Ĥ������Һ����_____(����������������С��)_______�ˡ�
���𰸡�CH4(g)+2NO2(g)= N2(g)+CO2(g)+2H2O(l) ��H=-955 kJ/mol �ٷ�Ӧ��Ļ��С�ڷ�Ӧ����ͬ�����¸�������Ӧ BC 0.65 0.0425mol/(L��min) 0.96 SO2+2H2O-2e- =SO42- +4H+ ���� 6.2
��������
��1��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O(l)�Ļ�ѧ����ʽΪ��CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(1)����֪����CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=574kJmol1����CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=1160kJmol1����H2O(g)=H2O(l) ��H=44.0kJmol1����˹���ɼ��㽫![]() �ɵã�
�ɵã�
��2���ٷ�Ӧ���ԽСԽ�����У���A��ͼ����������㷨����ͼ��P�㲻һ��Ϊͼ�����ߵ㣻 B��ͼ�������֪�¶ȸ���200�棬2O3(g)3O2(g)����Ӧ���г̶ȼӴ���ϵ����Ũ�ȼ�С��NO��SO2��ת�������¶������������ͣ� C�������������䣬����С��Ӧ�����ݻ���2O3(g)3O2(g)��ƽ��������У�����Ũ�����۷�Ӧ��NO(g)+O3(g)NO2(g)+O2(g)����Ӧ��NOת����Ϊ85%����Ӧ��NOΪ0.85mol����Ӧ��O3Ϊ0.85mol����Ӧ��SO2(g)+O3(g)SO3(g)+O2(g)����Ӧ��SO2��ת����30%����Ӧ�Ķ�������0.3mol����Ӧ��O3Ϊ0.3mol��2O3(g)3O2(g)�������ֽⷴӦ�ij���ռ�������������10%��Ϊ0.2mol������ϵ��ʣ��O3�����ʵ���=2.0mol0.85mol0.3mol0.2mol=0.65mol��NO�ķ�Ӧ����=![]() ��ƽ��״̬��O2���ʵ���=0.85mol+0.3mol+0.3mol=1.45mol��O3���ʵ���0.65mol����������ʣ�����ʵ���=0.7mol�����������������������ʵ���0.3mol���ݴ˼���ƽ�ⳣ����
��ƽ��״̬��O2���ʵ���=0.85mol+0.3mol+0.3mol=1.45mol��O3���ʵ���0.65mol����������ʣ�����ʵ���=0.7mol�����������������������ʵ���0.3mol���ݴ˼���ƽ�ⳣ����
��3���ٱ����Ƕ�������������ˮ��Ӧ�������ᣬM�缫Ϊ������N�缫Ϊ������M�缫�϶�������ʧȥ������������SO42������ԭ���غ�����غ��֪����ˮ�μӷ�Ӧ�������������ɣ������ӽ���Ĥ����������ͨ�������缫��ӦʽΪ��SO2+2H2O2e=SO42+4H+�������·ͨ��0.2mol����ʱ����������ͨ��0.1mol����������������0.2mol���ݴ˼��������仯��
��1��CH4(g)��NO2(g)��Ӧ����N2(g)��CO2(g)��H2O(l)�Ļ�ѧ����ʽΪ��CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(1)����֪����CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=574kJmol1����CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=1160kJmol1����H2O(g)=H2O(l) ��H=44.0kJmol1����˹���ɼ��㽫![]() =955kJ/mol���ɵã�CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(1) ��H=955kJ/mol
=955kJ/mol���ɵã�CH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(1) ��H=955kJ/mol
��2���ٷ�Ӧ��NO(g)+O3(g)NO2(g)+O2(g)��H1=200.9kJmol1Ea1=3.2kJmol-1
��Ӧ��SO2(g)+O3(g)SO3(g)+O2(g)��H2=241.6kJmol1 Ea2=58kJmol1
��Ӧ��Ļ��С�ڷ�Ӧ����ͬ�����¸�������Ӧ����ͬ�¶���NO��ת����Զ����SO2���ʴ�Ϊ����Ӧ��Ļ��С�ڷ�Ӧ����ͬ�����¸�������Ӧ��
��A��ͼ����������㷨����ͼ��P�㲻һ��Ϊͼ�����ߵ㣬��һ��Ϊƽ��㣬�����ǽ���ƽ��״̬�����е�һ�㣬��A����
B��ͼ�������֪�¶ȸ���200�棬2O3(g)
C�������������䣬����С��Ӧ�����ݻ���2O3(g)3O2(g)��ƽ��������У�����Ũ������Ӧ��ͷ�Ӧ��ƽ��������У�NO��SO2��ת��������C��ȷ��
�ʴ�Ϊ��BC��
�۷�Ӧ��NO(g)+O3(g)NO2(g)+O2(g) NOת����Ϊ85%����Ӧ��NOΪ0.85mol����Ӧ��O3Ϊ0.85mol����Ӧ��SO2(g)+O3(g)SO3(g)+O2(g)SO2��ת����30%����Ӧ�Ķ�������0.3mol����Ӧ��O3Ϊ0.3mol��2O3(g)3O2(g)�������ֽⷴӦ�ij���ռ�������������10%��Ϊ0.2mol������ϵ��ʣ��O3�����ʵ���=2.0mol0.85mol0.3mol0.2mol=0.65mol��NO�ķ�Ӧ����=0.85mol2L��10min=0.0425mol/(Lmin)��ƽ��״̬��O2���ʵ���=0.85mol+0.3mol+0.3mol=1.45mol��O3���ʵ���0.65mol����������ʣ�����ʵ���=0.7mol�����������������������ʵ���0.3mol��SO2(g)+O3(g)SO3(g)+O2(g)��ƽ�ⳣ��K= =0.96
=0.96
��3�������Ƕ�������������ˮ��Ӧ�������ᣬM�缫Ϊ������N�缫Ϊ������M�缫�϶�������ʧȥ������������SO42������ԭ���غ�͵���غ��֪����ˮ�μӷ�Ӧ�������������ɣ��缫��ӦʽΪ�� SO2+2H2O2e=SO42��+4H+�������·ͨ��0.2mol����ʱ������������ͨ��0.1mol�������������Ҳ�����0.2mol�������Һ�����������������=0.1mol��64g/mol0.2mol��1g/mol=6.2g��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���������ƣ�NaNO2����һ�ֳ�����ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������ij��ȤС���������ʵ��̽������������֪����
��2NO + Na2O2=2NaNO2
��2NO2+ Na2O2=2NaNO3
������KMnO4��Һ�ɽ�NO2������ΪNO3����MnO4����ԭ��Mn2+��
��.��Ʒ�Ʊ�����飺������װ���Ʊ�NaNO2��
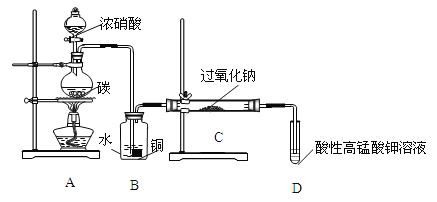
��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ________��
��2��Bװ�õ�������______________________________��
��3����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�Ʊ�����NaNO2Ӧ��B��Cװ�ü�����һ��װ�ã����ڿ��ڻ������ӵ�װ��ͼ��������ʢ�ŵ��Լ�________��

��4�������ʵ�����װ��C��NaNO2�Ĵ��ڣ�д������������ͽ��ۣ�________��
��.�����IJⶨ
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
����� | 1 | 2 | 3 | 4 |
KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
��5����һ��ʵ�����ݳ����쳣����������쳣��ԭ�������________��˫��ѡ��
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B����ƿϴ����δ����
C���ζ��������Ӷ���
D���ζ����˸��Ӷ���
��6�����ݱ������ݣ��������ù������������Ƶ���������____________________��
���������4λ��Ч���֣�