��Ŀ����
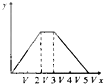
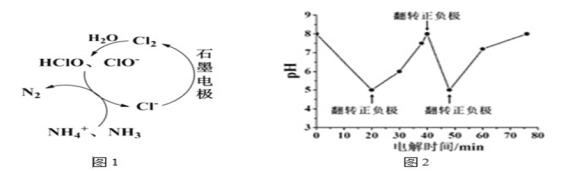
����Ŀ��������ˮ�еĵ�������Ҫ����κ���������ʽ���ڣ���������ʯī���缫���õ�ⷨ����Һ��ȥ�������ʱ����ͼ1ԭ����ʾ�ɽ��г�������Cl-����ʱ����Ҫ������Ч�ȣ�HClO��ClO-����NH4+��NH3����ΪN2����ת��Դ���������ɽ��г��ף�ԭ��������Fe2+��PO43-ת��ΪFe3(PO4)2������ͼ2Ϊij��Cl-��ˮ�ڵ��������ѳ���������ҺpH�ı仯������˵����ȷ���ǣ� ��
A.��ⷨ������Ч��HClO����NH4+�����ӷ���ʽΪ3HClO+2NH4+=3Cl-+N2��+3H2O+5H+
B.��ҺpHԽС��Ч��Ũ��Խ����ȥ����Խ��
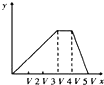
C.ͼ2��20~40min�ѳ���Ԫ������Ԫ�أ���ʱ�����缫��ӦʽΪ2H++2e-=H2��
D.ͼ2��0~20min�ѳ���Ԫ���ǵ�Ԫ�أ���ʱ��������
���𰸡�AC
��������
������Cl-����ʱ����Ҫ������Ч�ȣ�HClO��ClO-����NH4+��NH3����ΪN2��֪����Һ�д���Cl2+H2O![]() H++Cl-+HClO���Լ�����ʱ�ܷ�ӦΪ3HClO+2NH4+=3Cl-+N2��+3H2O+5H+��
H++Cl-+HClO���Լ�����ʱ�ܷ�ӦΪ3HClO+2NH4+=3Cl-+N2��+3H2O+5H+��
���ݳ���ԭ��������Fe2+��PO43-ת��ΪFe3(PO4)2��������Һ����Fe2+��˵��Fe��������ʧ���ӣ���Fe2+��
A����ⷨ������Ч��HClO��NH4+����ΪN2�����ӷ���ʽΪ 3HClO+2NH4+=3Cl-+N2��+3H2O+5H+��A��ȷ��
B������ҺpH���ͣ�c(H+)����Cl2+H2O![]() H++Cl-+HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½�����ȥ������pH�Ľ��Ͷ��½���B����
H++Cl-+HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½�����ȥ������pH�Ľ��Ͷ��½���B����
C������ʱ��Fe������ʧ���ӣ�ʯī�������������缫��ӦʽΪ2H++2e-=H2��H+�����ģ���ҺpH������ͼ2��20~40min��ҺpH�����ѳ���Ԫ������Ԫ�أ�C��ȷ��
D��ͼ2��0~20min��ҺpH�ı仯�ó��ѳ���Ԫ���ǵ�Ԫ�أ���ʱ����������D����
��ѡAC��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ����ϩ�Ȳ����������л�������������Ҫ����;����ҵ�ϳ����á��������ⷨ����ȡ��ϩ����Ӧ��ԭ��Ϊ��C3H8(g)![]() C3H6(g)+H2(g) ��H=+123kJ/mol
C3H6(g)+H2(g) ��H=+123kJ/mol
�ش��������⣺
��1����ʯ��ҵ�п�ͨ��___���ջ�ñ�ϩ�Ȳ�����������һ�ֹ��յ����ƣ���
��2����֪��
��ѧ�� | C-H | C-C | C=C | H-H |
���ܣ�kJ/mol�� | 412 | 348 | a | 436 |
���е�a=___��
��3����ҵ�Ͻ��и÷�Ӧʱ�����ڱ����в���ϡ��������Ϊϡ�ͼ���������ɱ�ķ�Ӧ�����У�ά�ֺ��£���ʹ��ѹǿ�㶨Ϊ0.1MPa������ϡ��������Ϊϡ�ͼ����ŵ���___���Դ�ƽ��Ƕȼ��Խ���___��
��4����ij�ܱ������г��������ı��飬��ñ����ת�������¶Ⱥ�ѹǿ�仯��ͼ��ʾ��
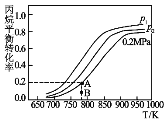
��ѹǿp1___p2�����������������������
��ͼ��A��ƽ�ⳣ��Kp=___����ƽ���ѹ����ƽ��Ũ�ȣ���ѹ����ѹ�����ʵ������������������λ��Ч���֣���
��B�����ﵽA����ʾ��ƽ��״̬���ڽ���ƽ�������v��___ v�������������������������
��д��һ����߱���ƽ��ת���ʵĴ�ʩ___��
����Ŀ�����ֳ���Ԫ�ص����ʻ�ṹ��Ϣ���±����Ը�����Ϣ�ش��й����⡣
Ԫ�� | A | B | C | D |
���ʽṹ ��Ϣ | ������ӹ���7���˶�״̬ | ԭ�ӵ�M����1�ԳɶԵ�p���� | һ�ֺ��ص�������Ϊ35��������Ϊ64 | �ж���ͬλ�أ�����һ�������������ԭ��������У |
(1)д��Bԭ�ӵĵ����Ų�ʽ___________��д��Cԭ�ӵĵ����Ų�ʽ___________
(2)����Ԫ���зǽ�������ǿ��Ԫ����____����������ǿ��Ԫ����_____����һ����������Ԫ����______��
(3)һ��������B��C���ʼ��ܷ�Ӧ����Ӧ�ķ�Ӧ����ʽΪ__________________________��