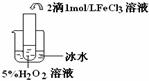
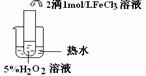
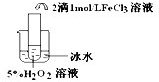
��Ŀ����
��ʢ���Ȼ�����Һ���Թ��е���������������Һ�����ɰ�ɫ���Ȼ����������������Թ�����백ˮ�������ܽ�,������ɫ��������[Ag��NH3��2]+������˵����ȷ���ǣ� ��
A. ��Һ��Cl-��Ag+�����ʵ�����Ӧǰ��
B. ��λ����ǿ�ȶ��ܴ��������ﶼ���ȶ�
C. ��λ�����ô���ͷ�ļ��߱�ʾ����һ������Ĺ��ۼ�
D. ��[Ag��NH3��2]+�����У�Ag+ �����¶Ե��ӣ�NH3�ṩ�չ��
C
��������

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д���ѧ����ʵ��Ϊ������ѧ�ƣ���ѧʵ�����ж���ʵ��Ͷ���ʵ��֮�֡�������ѧ֪ʶ������ʵ����з�������գ�
��.
| ʵ��Ŀ�� |
| ||
| ʵ�� |
|
|
|
| ʵ����� | ���ۣ� FeCl3��ʵ���е�����Ϊ�� | ||
| ��Ӧ����ʽ |
| ||
��.
| ʵ��Ŀ�� | ̽��±�ص��������Ե����ǿ�� | |
| ʵ�� | ���� | ���ӷ���ʽ |
| 1.��������ˮ�ֱ����ʢ��KBr��NaI��Һ���Թ��У������CCl4�������� | ��1����Һ�ֲ㣬�ϲ㼸����ɫ���²�Ϊ��ɫ�� ��2�� | ��1�� ��2�� |
| 2.��������ˮ����ʢ��NaI��Һ���Թ��У����������CCl4�������� | ��3�� | |
| ʵ����۲���ԭ�ӽṹ�Ͻ���ԭ�� |
| |
��.
���ϣ����������ڱ����Ȼ�����Һ��ʵ���ҿ�����MnO2��Ũ�����ڼ��ȵ��������Ʊ�����
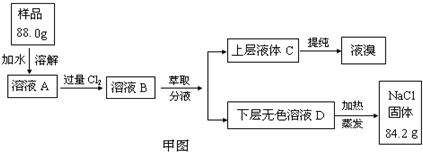
ʵ�飺ijʳ����Ʒ�л���NaBr���ʣ�Ϊ�ⶨ��ʳ����Ʒ�Ĵ��ȣ����ʵ��������ͼ��ʾ��

�Ķ��������Ϻ�ʵ����̣����������գ�
��1������·���ͼ��ѡ���ĸ�װ�ã����ظ�ʹ�ã����������ȡ������ҺA��ͨ�����Cl2��ʵ�飬����ѡװ�õ�ѡ��������뷽����������д��װ�������ŵĻ�ѧҩƷ��
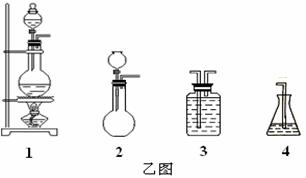

��2��Ϊ��֤����ͼ����ҺD�м���������Br������ѡ�õ��Լ�Ϊ ����ѡ����ĸ��
A. ��ˮ B. ���۵⻯����Һ
C. ������ˮ�����Ȼ�̼ D. ʳ��ˮ
��3�����ݼ�ͼ��ʾ������ȡ������Ӧѡȡ���л��ܼ�Ϊ
A. ���Ȼ�̼ B. ˮ C. �Ҵ� D. ��
��4������ȡ����Һ�Ĺ����У��ѷ�Һ©���������ϵİ��۶�©�����ϵ�С�ף���Ŀ����
��5���Լ���ԭ�����������Ȼ��Ƶ���������Ϊ %



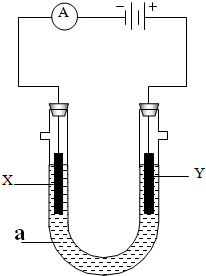 ʹ����ͼ��ʾװ�ý��е��ʵ�飬����X��Y��Ϊʯī�缫��U�ι���ʢ��200mL��Һa���Իش��������⣺
ʹ����ͼ��ʾװ�ý��е��ʵ�飬����X��Y��Ϊʯī�缫��U�ι���ʢ��200mL��Һa���Իش��������⣺