��Ŀ����
ij���Ͻ��к��кϽ�Ԫ��þ��ͭ���裬Ϊ�˲ⶨ�úϽ������ĺ������������ʵ�鷽������ش��й����⣺
��1����ȡ��Ʒa g������ʱӦ��ʹ�õ�����������_________��
��2������Ʒ����������ϡ���ᣬ���ˣ���Һ����Ҫ����________�������к���_______��
��3����Һ�м��������NaOH��Һ�����ˡ����������Ļ�ѧʽΪ_________��
��4�������裨3������Һ��ͨ��������CO2���壬���ˡ���Ӧ�����ӷ���ʽΪ_________��
��5�����裨4���������õ�����������ˮϴ��3�Σ���ɺ�����������������ټ���Ϊֹ���Ƶ�������Ϊb g����ԭ��Ʒ��������������Ϊ_________����a��b��ʾ����
��1��������ƽ��1�֣� ��2��MgCl2��AlCl3 Cu��Si����1�֣�
��3��Mg��OH��2��1�֣�
��4��AlO![]() +CO2+2H2O====Al��OH��3��+HCO
+CO2+2H2O====Al��OH��3��+HCO![]() ��3�֣�
��3�֣�
��5��![]() ��100%��3�֣�
��100%��3�֣�
����:
�������֪����������ƽ��ȡ��a g��Ʒ��ʵ����������������ת����ϵ��
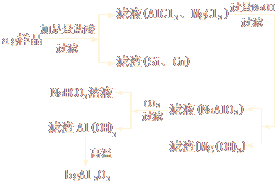
������Ʒ��Al����������Ϊ��
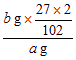 ��100%=
��100%=![]() ��100%
��100%

��ϰ��ϵ�д�
�����Ŀ