��Ŀ����
���й��ڵ������Һ��������ȷ����( )
A�������£���0.1 mol��L-1һԪ��BOH��Һ��pH=10������֪BOH��Һ���ڣ�BOH B++OH- B++OH- |
| B�������£�pH��7��NH4Cl�백ˮ�Ļ����Һ�У�c(Cl-)��c(NH4+)��c(H+)��c(OH-) |
| C���к�pH���������ͬ������ʹ�����Һ������NaOH�����ʵ�����ͬ |
| D����pH��4������ϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ����� |
A
����

��ϰ��ϵ�д�
�����Ŀ
���и���Һ�У�������ȷ����
| A��0.1mol��L��(NH4)2SO4��Һ�У�c(SO42��)��c(NH4��)��c(H��)��c(OH��) |
| B��25��ʱ����pH=3��������Һ��pH=11�İ�ˮ�������Ϻ���Һ������Ũ�ȵĴ�С��ϵ��c(Cl��)��c(NH4��)��c(OH��)��c(H��) |
| C����0.1mol/L������Һ�Ӵ���ˮϡ�ͺ���Һ��pH���� |
| D��Na2CO3��Һ�У�c(Na+ )="2c" (CO32- ) + c (HCO3- ) + c (H2CO3 ) |
һ���¶��£�±����AgX(X��Cl����Br����I��)��Ag2CrO4�ij����ܽ�ƽ��������ͼ��ʾ��������p(Ag+)��ʾ��-lgc(Ag+)����������Y��ʾ�� -lgc(X-)����-lgc(CrO42��)��������˵����ȷ����
| A��a���ʾc(Ag+)=c(CrO42��) |
| B��b��ɱ�ʾAgI�ı�����Һ |
| C�����¶���AgI��KspԼΪ1��10��16 |
| D�����¶���AgCl��AgBr������Һ�У�c(Cl��)��c(Br��) |
�����£�Ksp��CaSO4��=9´10��6��������CaSO4��ˮ�еij����ܽ�ƽ������ͼ������˵����ȷ���ǣ� ��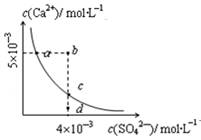
| A�����κ���Һ�У�c(Ca2+)��c(SO42��)����� |
| B��d����Һͨ���������Ա䵽c�� |
| C��a���Ӧ��Ksp����c���Ӧ��Ksp |
| D��b�㽫�г������ɣ�ƽ�����Һ��c(SO42��)һ������4´10��3 mol��L��1 |
����˵������ȷ����(����)
| A�����ܵ������ˮ��Һ�дﵽ�����ܽ�ƽ��ʱ,�������ܽ⼴ֹͣ |
| B��KspԽС,���ܵ������ˮ�е��ܽ�����һ��Խ�� |
| C��Ksp�Ĵ�С������Ũ����,ֻ�����ܵ���ʵ����ʺ��¶��й� |
| D����ͬ�¶���,AgCl��ˮ�е��ܽ���������NaCl��Һ�е���ͬ |
�±��г��˼��ֳ������ʵĵ��볣�����ܶȻ���
��һ������0.2 mol��L CaCl2��Һ�м���������������Һ�����Բ�����������( )
| A����ˮ�������c(H��)=10��9 mol/L��HF��Һ |
| B��pH��10�İ�ˮ |
| C��1 mol��L��NaHCO3��Һ |
| D��10-9 mol��L��AgNO3��Һ |
����pH��5��CH3COOH��Һ10 mL��Ҫʹ��pH����3���ɲ�ȡ�ķ�����( )
| A������Һ�м�ˮϡ����10 L |
| B������һ������NaOH���� |
| C������һ����pH��8��NaOH��Һ |
| D������һ��Ũ�ȵ����� |
��������10 mL pH=3�Ĵ�����Һ�м�ˮϡ�ͺ�����˵����ȷ����( )
| A����Һ�е������ӵ���Ŀ���� |
B����Һ��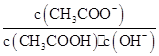 ���� ���� |
| C������ĵ���̶�����c(H+)������ |
| D���ټ���10 mL pH=11��NaOH��Һ�����ҺpH=7 |