��Ŀ����
����Ŀ��һѧ�����������ʵ�鷽������NaCl��CaCl2���ֹ�������
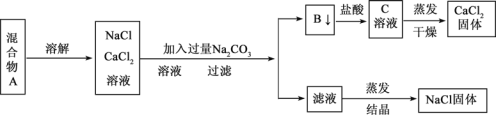
�ش���������
��1�������з�Ӧ�����ӷ���ʽ�� __________��________________��
��2������ʵ�鷽������õ���NaCl���������������ʣ�����Ϊ����������ijһ�����ë������һ������ȷ��Ʒ���Ӧ��____________________________��
��3����Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ��ɳ��������B ���ʺ���һ���ʵ�����������������__________________���������ʶ�Ӧ����ĸ��
��4��ʵ������м���ϴ�� B �����Ƿ�ϴ���ķ�����___________________��
��5����ʵ���������Ҫ0.1mol/L��Na2CO3��Һ450 mL��ʵ����ʦ��̼���ƾ���(Na2CO3��10H2O)���Ƹ�Ũ�ȵ���Һ������Э����ʦ�����������:
�� ����Na2CO3��Һʱ��Ҫ����Ҫ�����������ձ�����������___________________��
�� ��ʵ��ĵ�һ���Ǽ��㣬��ȡ_________g̼���ƾ��塣
�� ������ʱ���ӿ̶��ߣ��������Ƶ���ҺŨ��_____________���ƫ�ߡ���ƫ�͡����䡱����
���𰸡�Ca2+ + CO32- = CaCO3�� CaCO3 + 2H+ = Ca2+ + CO2��+ H2O ������Һ�м����������������ٲ���������������ᾧ A ȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ�� ��ͷ�ιܡ�500 mL ����ƿ 14.3 g ƫ��
��������
��1���������漰������Ӧ��һ����CaCl2��Na2CO3��Ӧ����һ����CaCO3�����ᷴӦ��
��2����Ϊ���ڳ���Ca2+��Na2CO3������������Һ�б�Ȼ����Na2CO3���ɼ���ȥ����
��3����Ϊ�ڷ�Ӧ������������NaCl��������Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ���������ԭ��Ʒ��������
��4������ϴ�� B �����Ƿ�ϴ���ķ����Ǽ���ϴ��Һ���Ƿ������Һ�е�ij�����ӡ�
��5����Ϊʵ����û��450 mL������ƿ������ѡ��500mL������ƿ��
�� ����Na2CO3��Һʱȱ�ٵ���������ʵ��������з�����
�� ����500mL������ƿ������ʱ��Һ�������Ӧ��500mL��
�� ������ʱ���ӿ̶��ߣ�����������Һ�����ƫ��Ũ�ȱ仯�����ù�ʽ���з�����
��1���������漰������Ӧ��һ����CaCl2��Na2CO3��Ӧ�����ӷ���ʽΪCa2+ + CO32- = CaCO3������һ����CaCO3�����ᷴӦ�����ӷ���ʽΪCaCO3 + 2H+ = Ca2+ + CO2��+ H2O����Ϊ��Ca2+ + CO32- = CaCO3����CaCO3 + 2H+ = Ca2+ + CO2��+ H2O��
��2����Ϊ���ڳ���Ca2+��Na2CO3������������Һ�б�Ȼ����Na2CO3���ɼ���ȥ������ȷ�IJ���Ϊ��������Һ�м����������������ٲ���������������ᾧ����Ϊ��������Һ�м����������������ٲ���������������ᾧ��
��3����Ϊ�ڷ�Ӧ������������NaCl��������Ҫ�ⶨ����Ʒ��NaCl��CaCl2�������ȣ���������ԭ��Ʒ����������Ϊ��A��
��4��ֻ�ܼ���ϴ��Һ���Ƿ����Cl-������Ϊȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ������Ϊ��ȡ���һ��ϴ��Һ�������еμ�ϡ�������������Һ�����ް�ɫ������������B������ϴ����
��5����Ϊʵ����û��450 mL������ƿ������ѡ��500mL������ƿ��
�� ����Na2CO3��Һʱ��ȱ�ٵ�����Ϊ��ͷ�ιܡ�500 mL ����ƿ����Ϊ����ͷ�ιܡ�500 mL ����ƿ��
��m(Na2CO3��10H2O)= 0.1mol/L��0.5L��286g/mol=14.3g������14.3 g��
�� ������ʱ���ӿ̶��ߣ�����������Һ�����ƫ�ߣ���c=![]() ��֪��Vƫ�ߣ���cƫ�͡���Ϊ��ƫ�͡�
��֪��Vƫ�ߣ���cƫ�͡���Ϊ��ƫ�͡�

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
