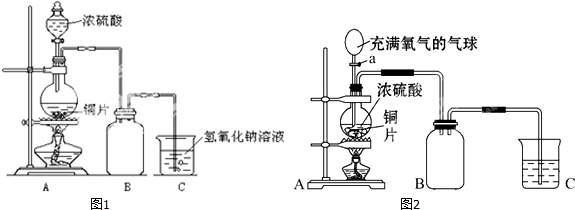
��Ŀ����
16��Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�飮��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ������������״������ͬ��Ϊb L����Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b=2��3��
���� ��1��������������������Һ����Ӧ����������������Һ��Ӧ����ƫ�����ƺ��������ݴ�д����Ӧ�ķ���ʽ��
��2�������������ڸ����������������������ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��3���ɷ���ʽ��֪����2�������ɵ�n��Fe���������ȼ���n��Al���������ᷴӦ���ɵ��������֮�ȵ��ڽ����ṩ�ĵ��ӵ����ʵ���֮�ȣ�ע���������ᷴӦ�����Ȼ�������
��� �⣺��1��������������������Һ����Ӧ����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2�������������ڸ�����������������������Ӧ����ʽΪ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3���ʴ�Ϊ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��3���ɷ���ʽ2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��֪����2�������ɵ��������ʵ���n��Fe���������ȼ����������ʵ���n��Al���������ᷴӦ���ɵ��������֮�ȵ��ڽ����ṩ�ĵ��ӵ����ʵ���֮�ȣ����ԣ�3�������ɵ������루1���������������֮��c��b=2n��Fe����3n��Al��=2��3��
�ʴ�Ϊ��2��3��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ���ȷ�Ӧԭ��Ϊ���ؼ���ע������ϡ���ᷴӦ���ɵ����������ӣ�Ϊ�״��㣬����������ѧ���ķ�����������ѧ����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� |  | B�� |  | C�� | 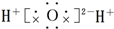 | D�� | 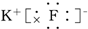 |
| A�� | CH4��Cl2���շ���ȡ����Ӧ | |
| B�� | 1��3-����ϩ������ʵ������巢����Ӧ | |
| C�� | ��ϩ���Ȼ���ӳ� | |
| D�� | �Ҵ����� |
| A�� | E��3s����E��3p����E��3d�� | B�� | E��3s����E��2s����E��1s�� | C�� | E��4f����E��3d����E��4s�� | D�� | E��5s����E��4s����E��4f�� |
| A�� | �ϳɰ���Ӧ�ڵ������ܹ��Է����У�����Ϊ��Ӧ�����֮�ʹ������������֮�� | |
| B�� | Ԫ�ط����ǿ���ȷ���������Ƿ���C��H��O��N��S��Cl��Br��Ԫ�أ�ԭ�����չ�����ȷ�������к�����Щ����Ԫ�� | |
| C�� | ��������Ԫ���������ڹ���Ԫ����Ѱ�Ҹ������������Ĵ������Խ��ͻ�ѧ��Ӧ�Ļ�ܣ��Ӷ��ܺõĽ���Ч�� | |
| D�� | ���߷ֱ���ӫ�������ܹ��۲쵽���׳߶ȵ����ʣ��������Ի�õ�������Һ�еķ���ͼ�� |
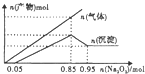
| A�� | �����Һ��m��Al3+��Ϊ5.4g | B�� | �����Һ��c��Mg2+��Ϊ0.5mol/L | ||
| C�� | �����Һ��pH=2 | D�� | �����Һ��c��Cl-��Ϊ1.7mol/L |