��Ŀ����
10����1���������������У�����һ�����ʵ�������������ֲ�ͬ��A��MgO��Na2O��CO2��CuO B��HCl��H2O��H2SO4��HNO3 C��NaOH��Na2CO3��KOH��Cu��OH��2
�������������ǣ��ѧʽ����ACO2��BH2O��CNa2CO3��
��2�����������ʣ���NaOH����CO2����NH4Cl����Na2O����MgCl2����Cu���������C2H5OH���ƾ�������������𣩣�
����ɷ��࣬���ڵ��ʵ��Ǣޣ�����������������Ǣڣ����ڼ�����������Ǣܣ���������Ǣߣ����ڼ��Ǣ٣������ε��Ǣۢݣ�
���Ƿ��ǵ���ʷ��࣬���ڵ���ʵ��Ǣ٢ۢܢݢߣ����ڷǵ���ʵ��Ǣڢ࣮
���� ��1��A��MgO��Na2O��CuO���ڽ��������
B��HCl��H2O��H2SO4��HNO3�����
C��NaOH��KOH��Cu��OH��2���ڼ
��2����������ͬ��Ԫ����ɵĴ����
�����������ǺͼӦ�����κ�ˮ�������
�����������Ǻ��ᷴӦ�����κ�ˮ�������
����ָ�������������ȫ���������ӵĻ����
����ָ�������������ȫ�������������ӵĻ����
����ָ�ܵ�����������ӣ���笠����ӣ���������ӵĻ����
��������ˮ������״̬���ܵ���Ļ������ǵ���ʣ�������ˮ������״̬�¶����ܵ���Ļ������Ƿǵ���ʣ�
��� �⣺��1��A��MgO��Na2O��CuO���ڽ��������CO2���ڷǽ��������
B��HCl��H2O��H2SO4��HNO3�����ᣬH2O���������
C��NaOH��KOH��Cu��OH��2���ڼNa2CO3�����Σ�
�ʴ�Ϊ��CO2��H2O��Na2CO3��
��2�����Cu�ǵ��ʣ�
CO2�ͼӦ�����κ�ˮ���������������
Na2O���ᷴӦ�����κ�ˮ���ڼ��������
����ָ�������������ȫ���������ӵĻ�����ʢ�H2SO4���ϣ�����ָ�������������ȫ�������������ӵĻ������NaOH���ϣ�
����ָ�ܵ�����������ӣ���笠����ӣ���������ӵĻ������NH4Cl��MgCl2���ϣ�
�ʴ�Ϊ���ޣ��ڣ��ܣ��ߣ��٣��ۢݣ�
��������ˮ������״̬���ܵ���Ļ������ǵ���ʣ���NaOH����NH4Cl����Na2O����MgCl2����������ϣ�������ˮ������״̬�¶����ܵ���Ļ������Ƿǵ���ʣ���CO2����C2H5OH���ƾ������ϣ�
�ʴ�Ϊ���٢ۢܢݢߣ��ڢ࣮
���� ���⿼�������ʵķ������⣬������ᡢ��Ρ�����ʡ��ǵ���ʵĶ��弰�������ݽ����жϣ��ѶȲ���

| A�� | CH4 | B�� | NH3 | C�� | H2 | D�� | C2H6 |
| A�� | �ڢ٢ۢݢܢޢ� | B�� | �ڢ٢ۢݢޢܢ� | C�� | �ڢ٢ݢۢޢܢ� | D�� | �٢ڢۢݢޢܢ� |
��1�������2������ԭ�Ӹ��� ��3�����ʵ�����4������ ��5���ܶȣ�
| A�� | ֻ�У�1����2����3�� | B�� | ֻ�У�1����2����4�� | C�� | ֻ�У�2����3����5�� | D�� | ��1����2����3����4����5�� |
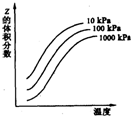 ��ͼ���¶Ⱥ�ѹǿ��X+Y?2Z��ӦӰ���ʾ��ͼ��ͼ���������ʾƽ����������Z���������������������ȷ���ǣ�������
��ͼ���¶Ⱥ�ѹǿ��X+Y?2Z��ӦӰ���ʾ��ͼ��ͼ���������ʾƽ����������Z���������������������ȷ���ǣ�������| A�� | X��Y��ֻ��һ��Ϊ��̬��ZΪ��̬ | B�� | X��Y��Z��Ϊ��̬ | ||
| C�� | �������淴Ӧ������ӦΪ���ȷ�Ӧ | D�� | �����¶ȣ�������Ӧ�����ƶ� |
 ��������������ǹ�ҵ����Ҫ�Ļ���ԭ�ϣ�Ҳ��ʵ�����ﳣ�����Լ���
��������������ǹ�ҵ����Ҫ�Ļ���ԭ�ϣ�Ҳ��ʵ�����ﳣ�����Լ���
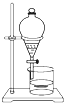
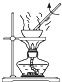
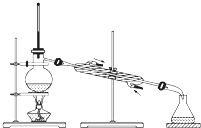
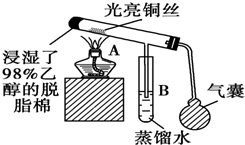 �йش�����������ɴӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ʵ��װ����ͼ��ʾ����ʵ�����Ϊ��Ԥ��ʹ���Ž��Ҵ�������ͼ��װ����������ͭ˿���м䲿�ּ��ȣ�Ƭ�̺�ʼ�н���ع�����������ɹ۲쵽���Ե�ʵ��������ش��������⣺
�йش�����������ɴӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ʵ��װ����ͼ��ʾ����ʵ�����Ϊ��Ԥ��ʹ���Ž��Ҵ�������ͼ��װ����������ͭ˿���м䲿�ּ��ȣ�Ƭ�̺�ʼ�н���ع�����������ɹ۲쵽���Ե�ʵ��������ش��������⣺